Zanamivir
If you find any inaccurate information, please let us know by providing your feedback here
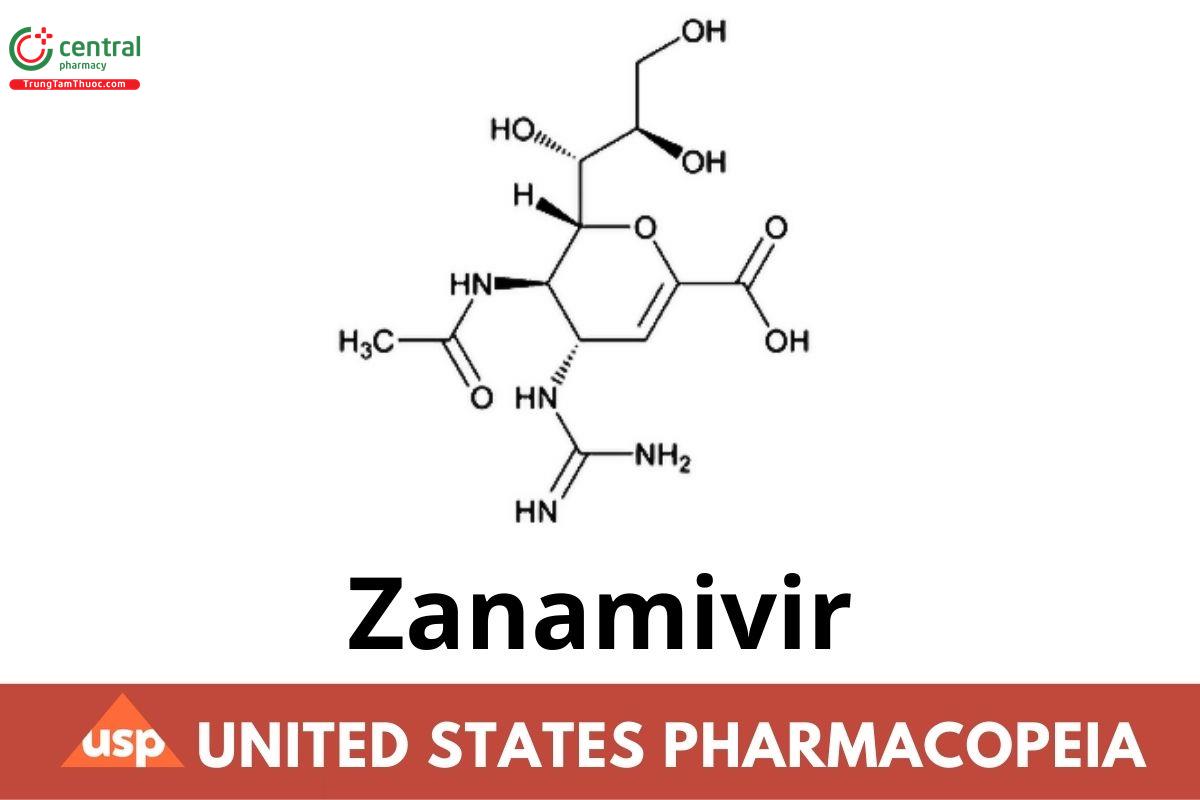
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C12H20N4O7 332.31
D-glycero-d-galacto-Non-2-enonic acid, 5-(acetylamino)-4-[(aminoiminomethyl)amino]-2,6-anhydro-3,4,5-trideoxy-;
5-Acetamido-2,6-anhydro-3,4,5-trideoxy-4-guanidino-d-glycero-d-galacto-non-2-enonic acid CAS RN: 139110-80-8; UNII: L6O3XI777I.
1 DEFINITION
Zanamivir contains NLT 98.0% and NMT 102.0% of zanamivir (C12H20N4O7), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K or 197M (CN 1-May-2020)
Wavenumber range: 4000 cm−1 to 400 cm−1
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Mobile phase: Acetonitrile and 7.5 mM sulfuric acid (60:40). Adjust with ammonia TS to a pH of 6.2.
Resolution solution: Prepare 2.5 µg/mL of talo-zanamivir and 0.05 mg/mL of zanamivir from USP Zanamivir Resolution Mixture RS in Mobile phase.
Standard solution: 0.045 mg/mL of USP Zanamivir RS in Mobile phase
Sample solution: 0.045 mg/mL of Zanamivir in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 234 nm
Column: 4.6-mm × 25-cm; 5-µm packing L82
Column temperature: 30°
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Samples: Resolution solution and Standard solution
Suitability requirements
Resolution: NLT 1.5 between talo-zanamivir and zanamivir, Resolution solution
Relative standard deviation: NMT 1.5%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of zanamivir (C12H20N4O7) in the portion of Zanamivir taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Zanamivir RS in the Standard solution (mg/mL)
Cu = concentration of Zanamivir in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
ORGANIC IMPURITIES
Mobile phase: Proceed as directed in the Assay.
System suitability solution: Prepare 0.45 mg/mL of zanamivir and 9 µg/mL each of imidazole, imidazole carboximidamide, zanamivir Urea analog, 4-amino zanamivir, 4-biguanide zanamivir, and talo-zanamivir from USP Zanamivir Related Compounds Mixture RS in Mobile phase.
Sample solution: 0.45 mg/mL of Zanamivir prepared as follows. Dissolve the sample using 40% of the final volume with water, and dilute with acetonitrile to volume.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 210 nm and 234 nm
Column: 4.6-mm x 25-cm; 5-µm packing L82
Column temperature: 30o
Flow rate: 1.5 mL/min
Injection volume: 20 µL
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 1.5 between the peaks of talo-zanamivir and zanamivir at. 234 nm
Analysis
Sample: Sample solution
Calculate the percentage of imidazole in the portion of Zanamivir taken:
Result = [(ri /F)/(ri /F + rz )] × 100
ri = peak response of imidazole at 210 nm
F = relative response factor for imidazole (see Table 1)
rz = peak response of zanamivir at 210 nm
Calculate the percentage of imidazole carboximidamide and 4-biguanide zanamivir in the portion of Zanamivir taken:
Result = [(ri /F)/(ri /F + rz )] × 100
ri = peak response of imidazole carboximidamide or 4-biguanide zanamivir at 234 nm
F = relative response factor for imidazole carboximidamide or 4-biguanide zanamivir (see Table 1)
rz = sum of the responses of all the peaks including the zanamivir peak at 234 nm
Calculate the percentage of O-triazinyl zanamivir, zanamivir urea analog, 4-amino zanamivir, talo-zanamivir, zanamivir dimer, and any other unspecied impurity in the portion of Zanamivir taken:
Result = (ru /rT ) × 100
ru = peak response of O-triazinyl zanamivir, zanamivir urea analog, 4-amino zanamivir, talo-zanamivir, zanamivir dimer, or any other unspecied impurity at 234 nm
rT = sum of the responses of all the peaks including the zanamivir peak at 234 nm
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Imidazolea | 0.26 | 2.5 | - |
| Imidazole carboximidamideb | 0.30 | 3.3 | 0.01 |
| O-Triazinyl zanamivirc | 0.60 | - | 0.3 |
| Zanamivir urea analogd | 0.70 | - | 0.2 |
| 4-Amino zanamivire | 0.77 | - | 0.2 |
| 4-Biguanide zanamivirf | 0.83 | 1.6 | 0.2 |
| Zanamivir | 1.00 | - | - |
| talo-Zanamivirg | 1.14 | - | - |
| Zanamivir dimerh | 2.75 | - | 0.5 |
| Any other unspecied impurity | - | - | 0.1 |
| Total impurities | - | - | 1.2 |
a 1H-Imidazole (No individual limit. Included in the determination of total impurities.)
b 1H-Imidazole-1-carboximidamide.
c 5-Acetamido-9-O-[4-amino-6-(1H-pyrazol-1-yl)-1,3,5-triazin-2-yl]-2,6-anhydro-3,4,5-trideoxy-4-guanidino-d-glycero-d-galacto-non-2-enonic acid.
d 5-Acetamido-9-O-[4-amino-6-(1H-pyrazol-1-yl)-1,3,5-triazin-2-yl]-2,6-anhydro-3,4,5-trideoxy-4-ureido-d-glycero-d-galacto-non-2-enonic acid.
e 5-Acetamido-9-O-[4-amino-6-(1H-pyrazol-1-yl)-1,3,5-triazin-2-yl]-2,6-anhydro-3,4,5-trideoxy-4-amino-d-glycero-d-galacto-non-2-enonic acid.
f 5-Acetamido-9-O-[4-amino-6-(1H-pyrazol-1-yl)-1,3,5-triazin-2-yl]-2,6-anhydro-3,4,5-trideoxy-4-(1-biguanidyl)-d-glycero-d-galacto-non-2-enonic acid.
g 5-Acetamido-2,6-anhydro-3,4,5-trideoxy-4-guanidino-d-glycero-d-talo-non-2-enonic acid (No individual limit. Included in the determination of total impurities.)
h 4,4'-(2-Amino-4-oxo-1,3,5-triazapent-2-ene-1,5-diyl) bis(5-acetamido-2,6-anhydro-3,4,5-trideoxy-d-glycero-d-galacto-non-2-enonic acid.)
5 SPECIFIC TESTS
Optical Rotation, Specific Rotation〈781S〉
Sample solution: 10 mg/mL in water
Acceptance criteria: +36.0° to +38.0°, measured at 20°, determined on the anhydrous and solvent-free basis
Water Determination, Method Ic〈921〉: 4.0%–9.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Store in a well-closed container at controlled room temperature.
USP Reference Standards 〈11〉
USP Zanamivir RS
USP Zanamivir Related Compounds Mixture RS
This Reference Standard is a mixture of zanamivir, imidazole, imidazole carboximidamide, zanamivir urea analog, 4-amino zanamivir, 4- biguanide zanamivir, talo-zanamivir, and zanamivir dimer. The chemical names are given below:
Imidazole: 1H-Imidazole.
Imidazole carboximidamide: 1H-Imidazole-1-carboximidamide.
Zanamivir urea analog: 5-Acetamido-9-O-[4-amino-6-(1H-pyrazol-1-yl)-1,3,5-triazin-2-yl]-2,6-anhydro-3,4,5-trideoxy-4-ureido-d-glycero-d-galactonon-2-enonic acid.
4-Amino zanamivir: 5-Acetamido-9-O-[4-amino-6-(1H-pyrazol-1-yl)-1,3,5-triazin-2-yl]-2,6-anhydro-3,4,5-trideoxy-4-amino-d-glycero-d-galacto-non-2-enonic acid.
4-Biguanide zanamivir: 5-Acetamido-9-O-[4-amino-6-(1H-pyrazol-1-yl)-1,3,5-triazin-2-yl]-2,6-anhydro-3,4,5-trideoxy-4-(1-biguanidyl)-d-glycero-dgalacto-non-2-enonic acid.
talo-Zanamivir: 5-Acetamido-2,6-anhydro-3,4,5-trideoxy-4-guanidino-d-glycero-d-talo-non-2-enonic acid.
Zanamivir dimer: 4,4'-(2-Amino-4-oxo-1,3,5-triazapent-2-ene-1,5-diyl) bis(5-acetamido-2,6-anhydro-3,4,5-trideoxy-d-glycero-d-galacto-non-2-enonic acid.)
USP Zanamivir Resolution Mixture RS
This Reference Standard is a mixture of zanamivir and talo-zanamivir.


