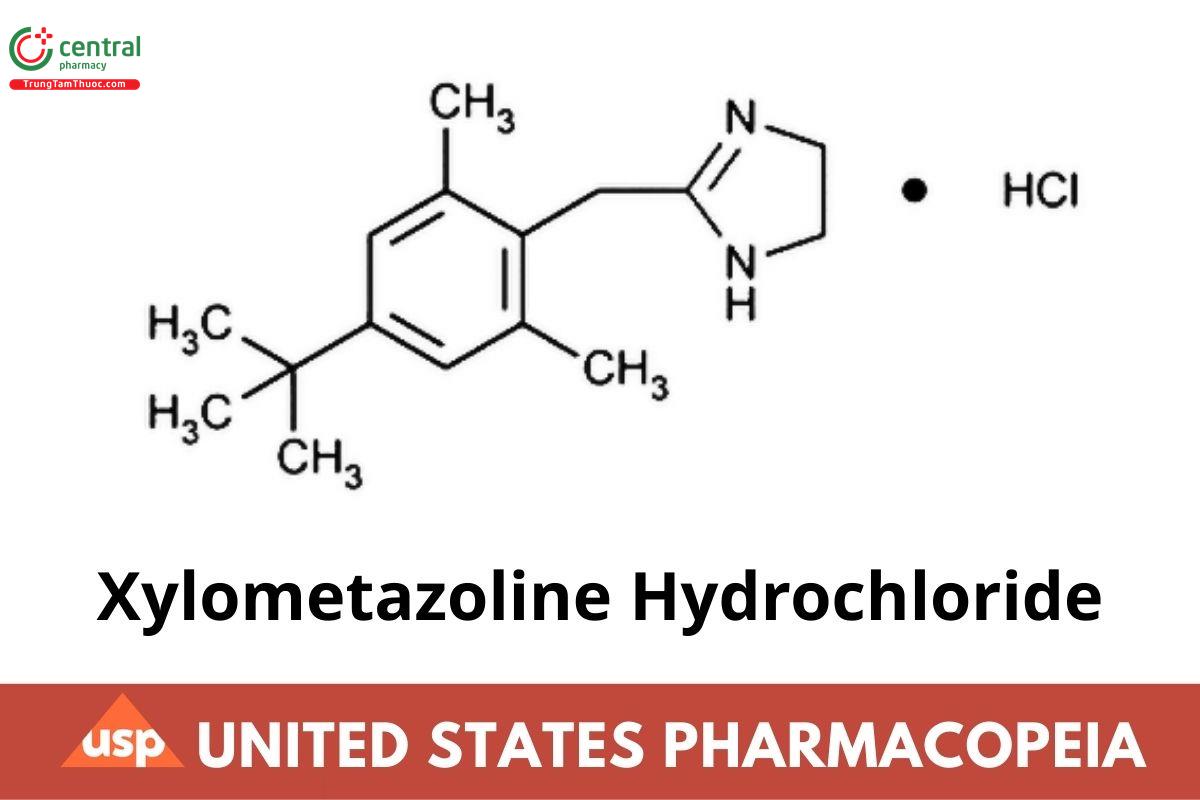
Xylometazoline Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C16H24N2 · HCl 280.84
1H-Imidazole, 2-[[4-(1,1-dimethylethyl)-2,6-dimethylphenyl]methyl]-4,5-dihydro-, monohydrochloride; 2-(4-tert-Butyl-2,6-dimethylbenzyl)-2-imidazoline monohydrochloride CAS RN: 1218-35-5; UNII: X5S84033NZ.
1 DEFINITION
Change to read:
Xylometazoline Hydrochloride contains NLT 98.0% and NMT 102.0% (USP 1-Aug-2019) of xylometazoline hydrochloride (C16H24N2 · HCl), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197M (CN 1-May-2020), (197K), or (1974) (USP 1-A00-2019)
Change to read:
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay (USP 1-Aug-2019)
Add the following:
C. IDENTIFICATION TESTS GENERAL (191), Chemical Identification Tests, Chloride
Sample solution: 5 mg/mL of Xylometazoline Hydrochloride in water
Acceptance criteria: Meets the requirements of the silver nitrate precipitate test (USP 1-Aug-2019)
3 ASSAY
Change to read:
PROCEDURE
Solution A: 1.4 g/L of monobasic potassium phosphate. Adjust with phosphoric acid to a pH of 3.0.
Solution B: Acetonitrile
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 70 | 30 |
| 5 | 70 | 30 |
| 20 | 15 | 85 |
| 35 | 15 | 85 |
| 37 | 70 | 30 |
| 45 | 70 | 30 |
System suitability solution: 0.02 mg/mL each of USP Xylometazoline Hydrochloride RS and USP Xylometazoline Related Compound A RS in water
Standard solution: 0.1 mg/mL of USP Xylometazoline Hydrochloride RS in water
Sample solution: 0.1 mg/mL of Xylometazoline Hydrochloride in water
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times are given in Table 2.]
Suitability requirements
Resolution: NLT 2.0 between xylometazoline related compound A and xylometazoline, System suitability solution
Tailing factor: NMT 2.0, Standard solution
Relative standard deviation: NMT 0.73%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of xylometazoline hydrochloride (C16H24N2 · HCl) in the portion of Xylometazoline Hydrochloride taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of xylometazoline from the Sample solution
rs = peak response of xylometazoline from the Standard solution
Cs = concentration of USP Xylometazoline Hydrochloride RS in the Standard solution (mg/mL)
Cu = concentration of Xylometazoline Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the dried basis (USP 1-Aug-2019)
4 IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.1%
Change to read:
ORGANIC IMPURITIES
Solution A, Solution B, Mobile phase, System suitability solution, and Chromatographic system: Proceed as directed in the Assay.
Sensitivity solution: 0.5 µg/mL of USP Xylometazoline Hydrochloride RS in water
Standard solution: 0.002 mg/mL of USP Xylometazoline Hydrochloride RS in water
Sample solution: 1.0 mg/mL of Xylometazoline Hydrochloride in water
System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
[NOTE-The relative retention times are given in Table 2.]
Suitability requirements
Resolution: NLT 2.0 between xylometazoline related compound A and xylometazoline, System suitability solution
Tailing factor: NMT 2.0, Standard solution
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Xylometazoline Hydrochloride taken:
Result = (ru /rs ) × (Cs /Cu ) × (1/F) × 100
ru = peak response of each impurity from the Sample solution
rs = peak response of xylometazoline from the Standard solution
Cs = concentration of USP Xylometazoline Hydrochloride RS in the Standard solution (mg/mL)
Cu = concentration of Xylometazoline Hydrochloride in the Sample solution (mg/mL)
F = relative response factor for the corresponding impurity (see Table 2)
Acceptance criteria: See Table 2. The reporting threshold is 0.05%.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Xylometazoline related compound A | 0.89 | 0.67 | 0.2 |
| Xylometazoline | 1.0 | - | - |
| Any individual unspecied impurity | - | 1.0 | 0.10 |
| Total impurities | - | - | 0.5 |
5 SPECIFIC TESTS
Change to read:
pH 〈791〉
Sample solution: 50 mg/mL of Xylometazoline Hydrochloride in water
Acceptance criteria: 5.0–6.6 (USP 1-Aug-2019)
Loss on Drying 〈731〉
Analysis: Dry at 105° for 4 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
Change to read:
USP Reference Standards 〈11〉
USP Xylometazoline Hydrochloride RS
USP Xylometazoline Related Compound A RS
N-(2-Aminoethyl)-2-[4-(tert-butyl)-2,6-dimethylphenyl]acetamide.
C16H26N2O 262.39 (USP 1-Aug-2019)


