Wheat Starch
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Wheat starch is obtained from the caryopsis of Triticum aestivum L. (T. vulgare Vill.).
2 IDENTIFICATION
A. Procedure
Analysis: Examine under a microscope using equal volumes of Glycerol and water.
Acceptance criteria: It presents large and small granules, and, very rarely, intermediate sizes. The large granules, usually 10–60 μm in diameter, are discoid or, more rarely, reniform when seen face-on. The central hilum and striations are invisible or barely visible, and the granules sometimes show cracks on the edges. Seen in profile, the granules are elliptical and fusiform and the hilum appears as a slit along the main axis. The small granules, rounded or polyhedral, are 2–10 μm in diameter. Between orthogonally oriented polarizing plates or prisms, the granules show a distinct black cross intersecting at the hilum.
B. Procedure
Sample solution: 20 mg/mL in water
Analysis: Boil for 1 min, and cool.
Acceptance criteria: A thin, cloudy mucilage is formed.
C. Procedure
Sample solution: 1 mL of the mucilage obtained in Identification B
Analysis: Add 0.05 mL of iodine and potassium iodide TS 2 to the Sample solution.
Acceptance criteria: A dark blue color is produced, which disappears upon heating.
3 IMPURITIES
Residue on Ignition 〈281〉
Sample: 1.0 g
Acceptance criteria: NMT 0.6%
Change to read:
3.1 Limit of Iron
Standard iron stock solution A: Equivalent to 10 μg/mL of iron prepared as directed under Iron 〈241〉, Procedures, Procedure 1 (CN 1-Jun-2023)
Standard iron stock solution B: 1 μg/mL of iron from Standard iron stock solution A in water. [Note—Prepare immediately before use.]
Standard iron solution: Transfer 10 mL of Standard iron stock solution B to a test tube, and add 2 mL of citric acid solution (2 in 10), and 0.1 mL of thioglycolic acid. Add 10 N ammonium hydroxide until the solution is distinctly alkaline to litmus, and dilute with water to 20 mL.
Sample solution: Shake 1.5 g of Wheat Starch with 15 mL of 2 N hydrochloric acid, and filter. Transfer 10 mL of the filtrate to a test tube, and add 2 mL of citric acid solution (2 in 10) and 0.1 mL of thioglycolic acid. Add 10 N ammonium hydroxide until the solution is distinctly alkaline to litmus, and dilute with water to 20 mL.
Acceptance criteria: After 5 min, any pink color in the Sample solution is not more intense than that in the Standard iron solution, corresponding to a limit of 10 ppm of iron.
3.2 Limit of Sulfur Dioxide
Carbon dioxide: Use carbon dioxide, with a flow regulator that will maintain a flow of 100 ± 5 mL/min.
Bromophenol blue indicator solution: 0.2 mg/mL of bromophenol blue in dilute alcohol. Filter if necessary.
Hydrogen peroxide solution: Dilute 30% hydrogen peroxide with water to obtain a 3% solution. Just before use, add 3 drops of Bromophenol blue indicator solution, and neutralize to a violet-blue endpoint with 0.01 N sodium hydroxide. Do not exceed the endpoint.
Apparatus: Figure 1
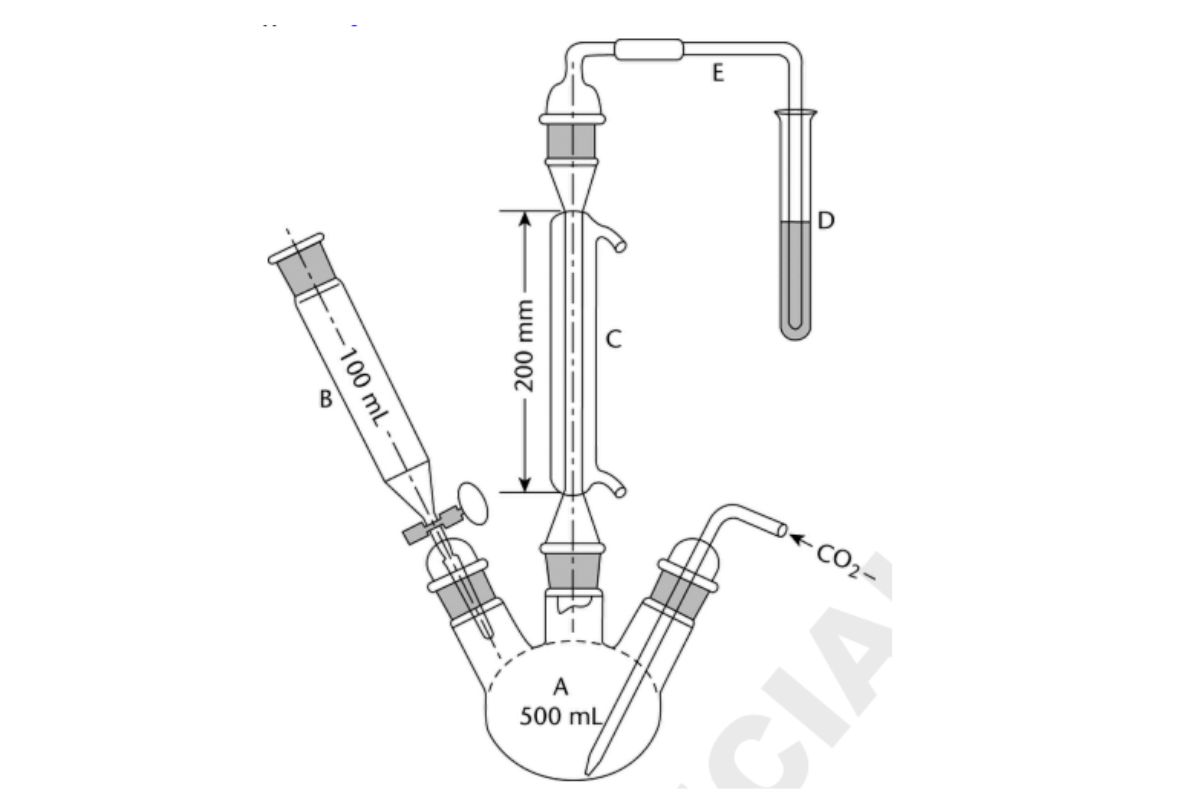
In this test, the sulfur dioxide is released from the sample in a boiling acid medium and is removed by a stream of carbon dioxide. The separated gas is collected in a dilute hydrogen peroxide solution where the sulfur dioxide is oxidized to sulfuric acid and titrated with standard alkali. The apparatus consists essentially of a 500-mL three-neck, round-bottom boiling flask, A; a separatory funnel, B, having a capacity of 100 mL or greater; a gas inlet tube of sufficient length to permit introduction of the carbon dioxide within 2.5 cm of the bottom of the boiling flask; a reflux condenser, C, having a jacket length of 200 mm; and a delivery tube, E, connecting the upper end of the reflux condenser to the bottom of a receiving test tube, D. Apply a thin film of stopcock grease to the sealing surfaces of all of the joints except the joint between the separatory funnel and the boiling flask, and clamp the joints to ensure tightness.
Sample: 25.0 g of Wheat Starch
Analysis: Add 150 mL of water to the boiling flask. Close the stopcock of the separatory funnel, and begin the flow of carbon dioxide at a rate of 100 ± 5 mL/min through the Apparatus. Start the condenser coolant flow. Add 10 mL of Hydrogen peroxide solution to a receiving test tube. After 15 min, without interrupting the flow of carbon dioxide, remove the separatory funnel from the boiling flask, and transfer the Sample into the boiling flask with the aid of 100 mL of water. Apply stopcock grease to the outer joint of the separatory funnel, and replace the separatory funnel in the boiling flask. Close the stopcock of the separatory funnel, and add 80 mL of 2 N hydrochloric acid to the separatory funnel. Open the stopcock of the separatory funnel to permit the hydrochloric acid solution to flow into the boiling flask, guarding against the escape of sulfur dioxide into the separatory funnel by closing the stopcock before the last few milliliters of hydrochloric acid drain out. Boil the mixture for 1 h. Remove the receiving test tube, and transfer its contents to a 200-mL wide-necked, conical flask. Rinse the receiving test tube with a small portion of water, add the rinsing to the 200-mL conical flask, and mix. Heat on a water bath for 15 min, and allow to cool. Add 0.1 mL of Bromophenol blue indicator solution, and titrate the contents with 0.1 N sodium hydroxide VS until the color changes from yellow to violet-blue. Perform a blank determination, and make any necessary correction (see Titrimetry 〈541〉).
Calculate the content, in ppm, of sulfur dioxide in the Sample taken:
Result = 1000 × 32.03 × (VN/W)
32.03 = milliequivalent weight of sulfur dioxide
V = volume of titrant consumed (mL)
N = normality of the titrant
W = weight of the Sample (g)
Acceptance criteria: NMT 50 ppm
3.3 Limit of Oxidizing Substances
Sample solution: Transfer 4.0 g to a glass-stoppered, 125-mL conical flask, and add 50.0 mL of water. Insert the stopper, and swirl for 5 min.
Transfer to a glass-stoppered, 50-mL centrifuge tube, and centrifuge to clarify. Transfer 30.0 mL of the clear supernatant to a glass-stoppered, 125-mL conical flask. Add 1 mL of glacial acetic acid and 0.5–1.0 g of potassium iodide. Insert the stopper, swirl, and allow to stand for 25–30 min in the dark. Add 1 mL of starch TS.
Analysis: Titrate with 0.002 N sodium thiosulfate VS to the disappearance of the starch–iodine color. Perform a blank determination, and make any necessary correction. Each milliliter of 0.002 N sodium thiosulfate is equivalent to 34 μg of oxidant, calculated as hydrogen peroxide.
Acceptance criteria: NMT 1.4 mL of 0.002 N sodium thiosulfate is required (20 ppm, calculated as H2O ).
4 SPECIFIC TESTS
Microbial Enumeration Tests 〈61〉 and Tests for Specified Microorganisms 〈62〉: The total aerobic microbial count does not exceed 103 cfu/g; the total combined molds and yeasts count does not exceed 102 cfu/g; and it meets the requirements of the test for the absence of Escherichia coli.
4.1 Loss on Drying 〈731〉
Sample: 1 g
Analysis: Dry the Sample at 130° for 90 min.
Acceptance criteria: NMT 15.0%
4.2 pH 〈791〉
Sample solution: Prepare a slurry by weighing 5.0 g of Wheat Starch, transferring to a suitable nonmetallic container, and adding 25.0 mL of freshly boiled and cooled water.
Analysis: Agitate continuously at a moderate rate for 1 min. Stop the agitation, and allow to stand for 15 min. Determine the pH to the nearest 0.1 unit.
Acceptance criteria: 4.5–7.0
4.3 Total Protein
Analysis: Weigh 3.0 g of sample; transfer to a combustion flask; add 4 g of a powdered mixture consisting of 100 g of potassium sulfate, 3 g of cupric sulfate, and 3 g of titanium dioxide; and add three glass beads. Wash any adhering particles from the neck of the flask with a fine jet of water. Add 25 mL of sulfuric acid, allowing it to run down the sides of the flask, and mix the contents by rotation. Close the mouth of the flask loosely, for example by means of a glass bulb with a short stem, to avoid excessive loss of sulfuric acid. Heat gradually at first, then increase the temperature until there is vigorous boiling with condensation of sulfuric acid in the neck of the flask; precautions should be taken to prevent the upper part of the flask from becoming overheated. Continue the heating until a clear solution is obtained and the inside wall of the flask is free from carbonaceous material. Cool, dissolve the solid material by cautiously adding 25 mL of water to the mixture, cool again, and place in a steam distillation apparatus. Add a volume of sodium hydroxide solution (42 in 100) suitable to make the color of the solution turn from bluish-green to brown or black, and distill immediately by passing steam through the mixture. Collect 40 mL of distillate in 50.0 mL of 0.01 N hydrochloric acid VS and enough water to cover the tip of the condenser. Toward the end of the distillation, fllower the receiver so that the tip of the condenser is above the surface of the acid and rinse the end of the condensing tube with a small quantity of water. Take precautions to prevent any water on the outer surface of the condenser from reaching the contents of the receiver. Titrate the distillate with 0.025 N sodium hydroxide VS, using methyl purple TS as the indicator (n mL of 0.025 N sodium hydroxide VS). Repeat the test without the substance to be examined in the combustion flask, using the same volume of sodium hydroxide solution (42 in 100) and titrating the distillate as for the sample determination (n mL of 0.025 N sodium hydroxide VS).
Content of nitrogen (%) = [0.03503 × (n2 − n1)]/m
n2 = amount of 0.025 N sodium hydroxide VS for the blank determination (mL)
n1 = amount of 0.025 N sodium hydroxide VS for the sample determination (mL)
m = amount of test substance weighed (g)
Acceptance criteria: NMT 0.3% of total protein (corresponding to 0.048% nitrogen, conversion factor: 6.25)
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. No storage requirements specified.


