Warfarin Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
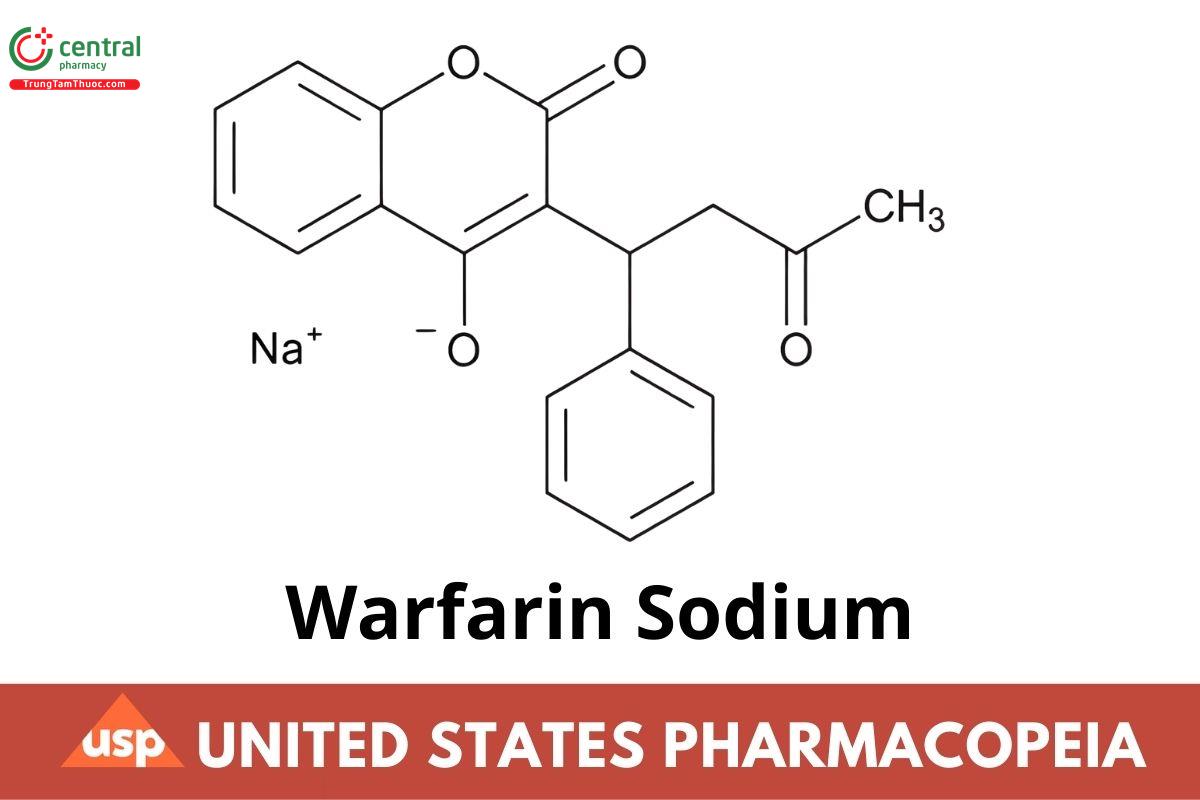
C19H15NaO4 330.31
2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1-phenylbutyl)-, sodium salt;
3-(α-Acetonylbenzyl)-4-hydroxycoumarin sodium salt,
Sodium 2-oxo-3-(3-oxo-1-phenylbutyl)-2H-chromen-4-olate CAS RN®: 129-06-6; UNII: 6153CWM0CL.
1 DEFINITION
Warfarin Sodium is an amorphous solid or a crystalline clathrate. The crystalline form consists principally of warfarin sodium and isopropyl alcohol in a 2:1 molecular ratio. It contains NLT 8.0% and NMT 8.5% of isopropyl alcohol. Warfarin Sodium contains NLT 97.0% and NMT 102.0% of warfarin sodium (C19H15NaO4), calculated on the anhydrous basis for the amorphous form or on the anhydrous and isopropyl alcohol-free basis for the crystalline form.
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K▲(CN 1-MAY-2020)
Standard: Use USP Warfarin RS.
Sample: Dissolve 100 mg in 25 ml of water, and adjust with hydrochloric acid to a pH of less than 3, using short-range pH indicator paper. Stir the mixture, and allow the precipitate to coagulate. Filter the mixture, wash the precipitate with four, 5-mL portions of water, and dry under vacuum over phosphorus pentoxide for 4 h. Use the warfarin obtained.
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
C.
Methoxyphenylacetic reagent: Dissolve 2.7 g of methoxyphenylacetic acid in 6 mL of 10% tetramethylammonium hydroxide aqueous solution, and add 20 mL of absolute alcohol.
Diluted ammonia: Dilute 41 g of ammonium hydroxide with water to 100 mL.
Ammonium carbonate solution: 158 mg/mL of ammonium carbonate in water
Sample solution: Dissolve 30 mg of Warfarin Sodium in 0.5 mL of water.
Analysis: Add 1.5 mL of Methoxyphenylacetic reagent to the Sample solution, and cool in ice water for 30 min.
Acceptance criteria: A voluminous, white, crystalline precipitate is formed. Place the precipitate in a water bath at 20° and stir for 5 min. The precipitate does not disappear. Add 1 mL of Diluted ammonia. The precipitate dissolves completely. Add 1 ml. of Ammonium carbonate solution. No precipitate is formed.
3 ASSAY
PROCEDURE
Buffer: Transfer 1.36 g of monobasic potassium phosphate to a 200-ml volumetric flask, and dissolve in 50 ml of water. Add 39.1 mL of 0.2 N sodium hydroxide, and dilute with water to volume. Adjust with sodium hydroxide or phosphoric acid to a pH of 7.4 ± 0.1.
Mobile phase: Methanol, glacial acetic acid, and water (64:1:36)
Standard stock solution: 0.376 mg/mL of USP Warfarin RS prepared as follows. Transfer USP Warfarin RS to a suitable volumetric flask, and dissolve in 0.1 N sodium hydroxide equivalent to 39% of the final volume. Add 0.2 M monobasic potassium phosphate, equivalent to 25% of the final volume, and dilute with water to volume.
Standard solution: Transfer 5 mL of Standard stock solution and 15 mL of Buffer into a conical flask, and mix.
Sample stock solution: 0.4 mg/mL of Warfarin Sodium prepared as follows. Transfer 100 mg of Warfarin Sodium, accurately weighed, to a 250-mL. volumetric flask, and dissolve in 97.8 mL of 0.1 N sodium hydroxide. Add 62.5 mL of 0.2 M monobasic potassium phosphate, and dilute with water to volume.
Sample solution: Transfer 5 mL of Sample stock solution and 15 mL of Buffer into a conical flask, and mix.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm x 25-cm; packing L7
Flow rate: 1.4 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 1.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of warfarin sodium (C19H15NaO4) in the portion of Warfarin Sodium taken:
Result = (rU/rS) × (CS/CU) x (Mr1/Mr2) × 100
rU = peak response of warfarin from the Sample solution
rS = peak response of warfarin from the Standard solution s
CS = concentration of USP Warfarin RS in the Standard solution ( mg/mL)
CU = concentration of Warfarin Sodium in the Sample solution (mg/mL)
Mr1 = molecular weight of warfarin sodium, 330.31
Mr2 = molecular weight of warfarin, 308.33
Acceptance criteria: 97.0%-102.0% on the anhydrous basis for the amorphous form or on the anhydrous and isopropyl alcohol-free basis for the crystalline form
4 IMPURITIES
ORGANIC IMPURITIES
Diluent: Methanol and water (25:75)
Mobile phase: Acetonitrile, glacial acetic acid, and water (32:1:68)
Standard stock solution: 0.12 mg/mL each of USP Warfarin RS and USP Warfarin Related Compound A RS prepared as follows. Transfer USP Warfarin RS and USP Warfarin Related Compound A.RS to a suitable volumetric flask, and add 0.1 N sodium hydroxide and methanol equivalent to 2% and 25% of the final volume, respectively. Dilute with water to volume.
Standard solution: 2.4 µg/mL each of USP Warfarin RS and USP Warfarin Related Compound A RS in Diluent, from Standard stock solution
Sample solution: 0.8 mg/mL of Warfarin Sodium in Diluent
System suitability solution: 2.4 µg/mL of USP Warfarin Related Compound A RS and 0.8 mg/mL of Warfarin Sodium in Diluent prepared as follows. Transfer 0.5 mL of Standard stock solution to a 25-mL volumetric flask and dilute with Sample solution to volume.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 260 nm
Column: 4.6-mm x 25-cm; packing 110
Column temperature: 35°
Flow rate: 1.5 mL/min
Injection volume: 50 µL
Run time: NLT 2 times the retention time of the warfarin peak
System suitability
Samples: Standard solution and System suitability solution
Suitability requirements
Resolution: NLT 3 between warfarin and warfarin related compound A peaks, System suitability solution
Relative standard deviation: NMT 5.0% for the warfarin and warfarin related compound A peaks, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of Alice's ketone (sodium salt of warfarin related compound A) in the portion of Warfarin Sodium taken:
Result = (rU/rS) × (CS/CU) x (Mr1/Mr2) × 100
rU = peak response of warfarin related compound A from the Sample solution
rS = peak response of warfarin related compound A from the Standard solution
CS = concentration of USP Warfarin Related Compound A RS in the Standard solution (mg/mL)
CU = concentration of Warfarin Sodium in the Sample solution (mg/mL)
Mr1 = molecular weight of Alice's ketone, 286.30
Mr2 = molecular weight of warfarin related compound A, 264.32
Calculate the percentage of any other individual impurity in the portion of Warfarin Sodium taken:
Result = (rU/rS) × (CS/CU) x (Mr1/Mr2) × (1/F) × 100
rU = peak response of any other individual impurity from the Sample solution
rS = peak response of warfarin from the Standard solution
CS = concentration of USP Warfarin RS in the Standard solution (mg/mL)
CU = concentration of Warfarin Sodium in the Sample solution (mg/mL)
Mr1 = molecular weight of warfarin sodium, 330.31
Mr2 = molecular weight of warfarin, 308.33
F = relative response factor for each individual impurity (see Table 1)
Acceptance criteria: See Table 1. Disregard any impurity peak less than 0.06%.
Table 1
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| 4-Hydroxycoumarina | 0.4 | 2.0 | 0.3 |
| Benzalacetoneb | 0.6 | 2.0 | 0.3 |
| Warfarin | 1.0 | — | — |
| Alice's ketonec | 1.2 | 1.0 | 0.3 |
| Any individual unspecified impurity | — | 1.0 | 0.10 |
| Total impurities | — | — | 1.0 |
a 4-Hydroxy-2H-chromen-2-one.
b (E)-4-Phenylbut-3-en-2-one.
c Sodium salt of warfarin related compound A; 3-(o-Hydroxyphenyl)-5-phenyl-2-cyclohexen-1-one sodium salt.
5 SPECIFIC TESTS
5.1 ISOPROPYL ALCOHOL CONTENT (CRYSTALLINE CLATHRATE FORM)
Internal standard solution: 4.25 mg/mL of n-propyl alcohol in water
Standard stock solution: 4.25 mg/mL of isopropyl alcohol in water
Standard solution: Transfer 2.0 mL of the Standard stock solution and 2.0 mL of the Internal standard solution to a headspace vial, seal, and mix.
Sample solution: Transfer 100 mg of Warfarin Sodium, 2.0 mL of water, and 2.0 mL of the Internal standard solution to a headspace vial, seal, and mix.
Blank solution: Transfer 2.0 mL of water and 2.0 mL of the Internal standard solution to a headspace vial, seal, and mix.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32 - mm x 30 - m 1.8-um coating of phase G43
Temperatures
Injector: 140°
Detector: 240°
Column: See Table 2.
Table 2
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 40 | 0 | 40 | 20 |
| 40 | 10 | 240 | 10 |
Carrier gas: Nitrogen
Flow rate: 1 mL/min
Injector type: Headspace; split ratio, 80:1
Temperatures
Equilibration: 65°
Needle: 75°
Transfer line: 85°
Headspace carrier pressure: 15 psi
Times
Equilibration: 20 min
Pressurization: 3.0 min
Loop fill: 0.2 min
Injection: 0.1 min
System suitability
Sample: Standard solution
[NOTE-The relative retention times for isopropyl alcohol and n-propyl alcohol are about 0.66 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 5.0 between isopropyl alcohol and n-propyl alcohol
Tailing factor: NMT 1.3 for the isopropyl alcohol peak
Relative standard deviation: NMT 2.0%, peak response ratio of isopropyl alcohol to n-propyl alcohol
Analysis
Samples: Standard solution, Sample solution, and Blank solution
Calculate the percentage of isopropyl alcohol in the portion of Warfarin Sodium taken:
Result = (RU/RS) × (CS/CU) × 100
RU = peak response ratio of isopropyl alcohol to n-propyl alcohol from the Sample solution
RS = peak response ratio of isopropyl alcohol to n-propyl alcohol from the Standard solution
CS = concentration of isopropyl alcohol in the Standard solution (mg/mL)
CU = concentration of Warfarin Sodium in the Sample solution (mg/mL)
Acceptance criteria: 8.0%-8.5%
5.2 PH (791)
Sample solution: 10 mg/mL
Acceptance criteria: 7.2-8.3
5.3 WATER DETERMINATION (921), Method
NMT 4.5% for the amorphous form; NMT 0.3% for the crystalline clathrate form
5.4 ABSORBANCE IN ALKALINE SOLUTION
Sample solution: 125 mg/mL in sodium hydroxide solution (1 in 20). Pass through a membrane filter.
Blank: Sodium hydroxide solution (1 in 20)
Analysis: Determine the absorbance of the solution in a 1-cm cell at 385 nm, with a suitable spectrometer, within 15 min.
Acceptance criteria: NMT 0.1
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed, light-resistant containers.
LABELING: Label it to indicate whether it is the amorphous or the crystalline form.
USP REFERENCE STANDARDS (11)
USP Warfarin RS
USP Warfarin Related Compound A RS
3-(o-Hydroxyphenyl)-5-phenyl-2-cyclohexen-1-one.
C18H16O2 264.32


