Vancomycin Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
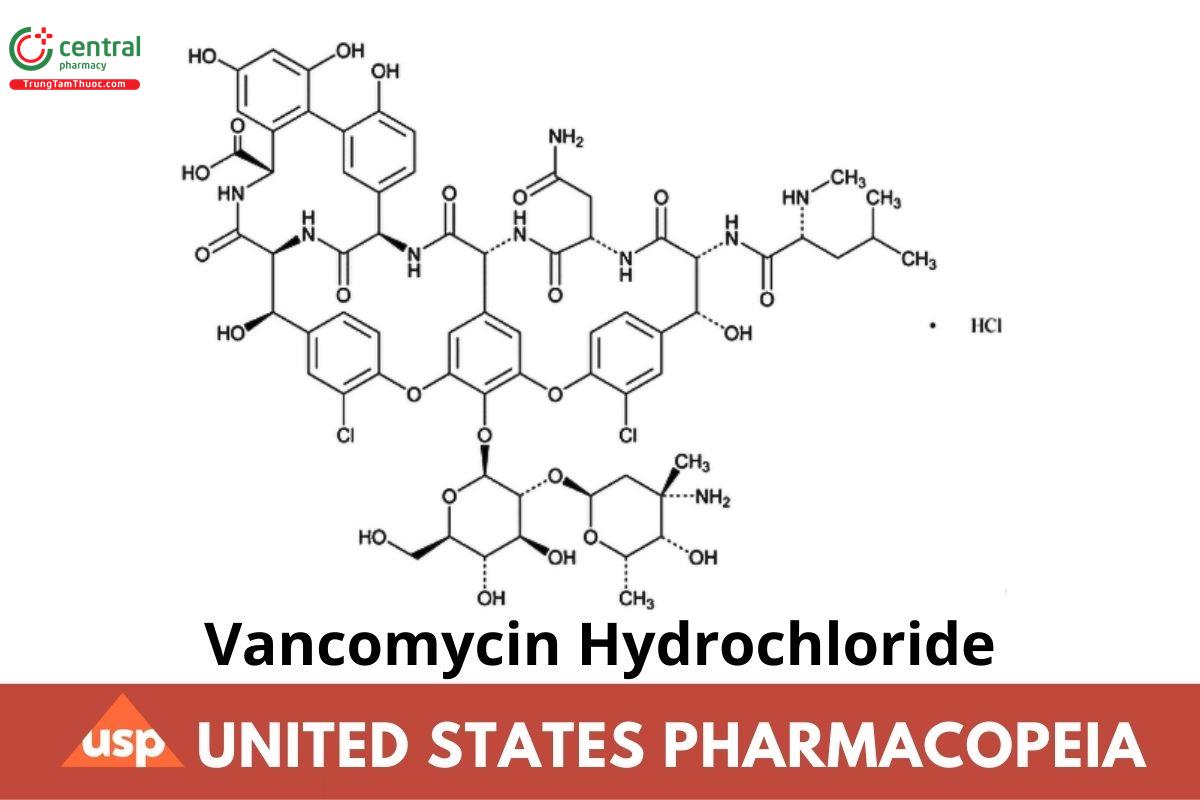
C₆₆H₇₆Cl₂N₉O₂₄ · HCl 1485.71
Vancomycin, monohydrochloride;
Vancomycin monohydrochloride;
(S )-(3S,6R,7R,22R,23S,26S,36R,38aR)-44-[[2-O-(3-Amino-2,3,6-trideoxy-3-C-methyl-α-l-lyxo-hexopyranosyl)-β-d-glucopyranosyl]oxy]-3-(carbamoylmethyl)-10,19-dichloro-2,3,4,5,6,7,23,24,25,26,36,37,38,38a-tetradecahydro-7,22,28,30,32-pentahydroxy-6-[(2R)-4-methyl-2-(methylamino)]valeramido]-2,5,24,38,39-pentaoxo-22H-8,11:18,21-dietheno-23,36-(iminomethano)-13,16:31,35-dimetheno-1H,16H-[1,6,9]oxadiazacyclohexadecino[4,5-m][10,2,16]benzoxadiazacyclotetracosine-26-carboxylic acid, monohydrochloride;
[3S-[3R*,6S*(S*),7S*,22S*,23R*,26R*,36S*,38aS*]]-3-(2-Amino-2-oxoethyl)-44-[[2-O-(3-amino-2,3,6-trideoxy-3-C-methyl-α-l-lyxo-hexopyranosyl)-β-d-glucopyranosyl]oxy]-10,19-dichloro-2,3,4,5,6,7,23,24,25,26,36,37,38,38a-tetradecahydro-7,22,28,30,32-pentahydroxy-6-[[4-methyl-2-(methylamino)-1-oxopentyl]amino]-2,5,24,38,39-pentaoxo-22H-8,11:18,21-dietheno-23,36-(iminomethano)-13,16:31,35-dimetheno-1H,16H-[1,6,9]oxadiazacyclohexadecino[4,5-m][10,2,16]-benzoxadiazacyclotetracosine-26-carboxylic acid, monohydrochloride; CAS RN®: 1404-93-9; UNII: 71WO621TJD.
1 DEFINITION
Vancomycin Hydrochloride is the hydrochloride salt of a kind of vancomycin, a substance produced by the growth of Streptomyces orientalis (Fam. Streptomycetaceae), or a mixture of two or more such salts. It has a potency equivalent to NLT 900 µg/mg of vancomycin, calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
3 ASSAY
Procedure: Proceed with Vancomycin Hydrochloride as directed under Antibiotics-Microbial Assays 〈81〉.
Acceptance criteria: NLT 900 µg/mg on the anhydrous basis
4 IMPURITIES
4.1 Organic Impurities
4.1.1 Procedure: Limit of Monodechlorovancomycin
[Note-The System suitability solution, Standard solution, and Sample solution should be refrigerated immediately after preparation and during analysis. The solutions are stable for 4 days when refrigerated.]
Mobile phase: Dissolve 2.2 g of 1-heptanesulfonic acid sodium salt in about 500 mL of water, add 125 mL of acetonitrile and 10 mL of acetic acid, and dilute with water to 1 L.
Rinse solution: 10% acetonitrile in water. [Note-Use to rinse needle and column.]
System suitability solution: Equivalent to 1 mg/mL of vancomycin B from USP Vancomycin B with Monodechlorovancomycin RS in water
Standard solution: Equivalent to 50 µg/mL of vancomycin B, from System suitability solution
Sample solution: 1 mg/mL of Vancomycin Hydrochloride in water
Blank: Water
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 280 nm
- Column: 4.6 mm × 25 cm; packing L1
- Column temperature: 60°
- Autosampler temperature: 5°
- Flow rate: 1.5 mL/min
- Injection volume: 50 µL
- [Note-This procedure is sensitive to temperature changes. Sufficient tubing should be placed in the column oven to ensure that the samples have reached 60° before separation.]
System suitability
- Samples: System suitability solution, Standard solution, Sample solution, and Blank
- [Note-Blank and Standard solution run times are about 90 min. System suitability solution and Sample solution run times are about 120 min.]
- [Note-The relative retention times for vancomycin B and monodechlorovancomycin are 1.0 and 1.1, respectively.]
- Suitability requirements
- Selectivity: The chromatogram for the Blank does not contain peaks that interfere with vancomycin B or monodechlorovancomycin.
- Retention times: ±3.0% between monodechlorovancomycin in the Sample solution and the mean of the monodechlorovancomycin peaks in the Standard solution
- Resolution: NLT 1.5 between vancomycin B and monodechlorovancomycin, System suitability solution
- Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of monodechlorovancomycin in the portion of Vancomycin Hydrochloride taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × P × 100
rᵤ = peak area of monodechlorovancomycin in the Sample solution
rₛ = average peak area of the vancomycin B peak in the Standard solution
Cₛ = concentration of USP Vancomycin B with Monodechlorovancomycin RS in the Standard solution (mg/mL)
Cᵤ = concentration of Vancomycin Hydrochloride in the Sample solution (mg/mL)
P = potency of vancomycin B in USP Vancomycin B with Monodechlorovancomycin RS (mg/mg)
Acceptance criteria: NMT 4.7% of monodechlorovancomycin is found.
5 SPECIFIC TESTS
5.1 Sterility Tests 〈71〉
Where the label states that Vancomycin Hydrochloride is sterile, it meets the requirements when tested as directed for Test for Sterility of the Product to Be Examined, Membrane Filtration, except to dissolve the specimen in water, instead of in Fluid A.
5.2 Bacterial Endotoxins Test 〈85〉
Where the label states that Vancomycin Hydrochloride is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.33 USP Endotoxin Unit/mg of vancomycin.
5.3 pH 〈791〉
2.5–4.5, 50 mg/mL in water
5.4 Water Determination, Method I 〈921〉
NMT 5.0%
5.5 Composition of Vancomycin
Solution A: Triethylamine and water (1:500). Adjust with phosphoric acid to a pH of 3.2.
Solution B: Acetonitrile, tetrahydrofuran, and Solution A (7:1:92)
Solution C: Acetonitrile, tetrahydrofuran, and Solution A (29:1:70)
Mobile phase: See the gradient table below. [Note-Make adjustments, if necessary, changing the acetonitrile proportion in Solution B to obtain a retention time of 7.5–10.5 min for the main vancomycin peak.]
| Time (min) | Solution B (%) | Solution C (%) |
| 0 | 100 | 0 |
| 12 | 100 | 0 |
| 20 | 0 | 100 |
| 22 | 0 | 100 |
| 23 | 100 | 0 |
| 30 | 100 | 0 |
System suitability solution: 0.5 mg/mL of USP Vancomycin Hydrochloride RS in water. Heat at 65° for 48 h, and allow to cool.
Sample solution 1: 10 mg/mL of Vancomycin Hydrochloride in Solution B
Sample solution 2: 0.4 mg/mL of Vancomycin Hydrochloride, from Sample solution 1 in Solution B
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 280 nm
- Column: 4.6-mm × 25-cm; 5-µm packing L1
- Flow rate: 2 mL/min
- Injection size: 20 µL
System suitability
- Sample: System suitability solution
- [Note-The elution order is compound 1, vancomycin B, and compound 2. Compound 2 elutes 3–6 min after the start of the period when the percentage of Solution C increases from 0%–100%.]
- Suitability requirements
- Resolution: NLT 3.0 between compound 1 and vancomycin B
- Column efficiency: NLT 1500 theoretical plates, calculated from the vancomycin B peak
Analysis
Samples: Sample solution 1 and Sample solution 2
[Note-Where baseline separation is not achieved, peak areas are defined by vertical lines extended from the valleys between the peaks to the baseline. The main component peak may include a fronting shoulder, which is attributed to monodechlorovancomycin. This shoulder should not be integrated separately.]
[Note-Correct any peak observed in the chromatograms obtained from Sample solution 1 and Sample solution 2 by subtracting the area response of any peak observed in the chromatogram of Solution B at the corresponding retention time.]
Measure the area responses for all of the peaks. Calculate the percentage of vancomycin B in the portion of Vancomycin Hydrochloride taken:
Result = [(D × rB)/((D × rB) + rA)] × 100
D = dilution factor, Sample solution 1 to Sample solution 2, 25
rB = corrected area response of the main peak of Sample solution 2
rA = sum of the corrected area responses of all the peaks, other than the main peak, from Sample solution 1
Calculate the percentage of each other peak in the portion of Vancomycin Hydrochloride taken:
Result = {rᵢ/[(D × rB) + rA]} × 100
rᵢ = corrected area response of any individual peak, other than the main peak of Sample solution 1
D = dilution factor, Sample solution 1 to Sample solution 2, 25
rB = corrected area response of the main peak of Sample solution 2
rA = sum of the corrected area responses of all the peaks, other than the main peak, from Sample solution 1
Acceptance criteria: NLT 85.0% of vancomycin B; NMT 5.0% of any peak other than the main peak
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
USP Reference Standards 〈11〉
USP Vancomycin Hydrochloride RS
USP Vancomycin B with Monodechlorovancomycin RS


