Valganciclovir Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
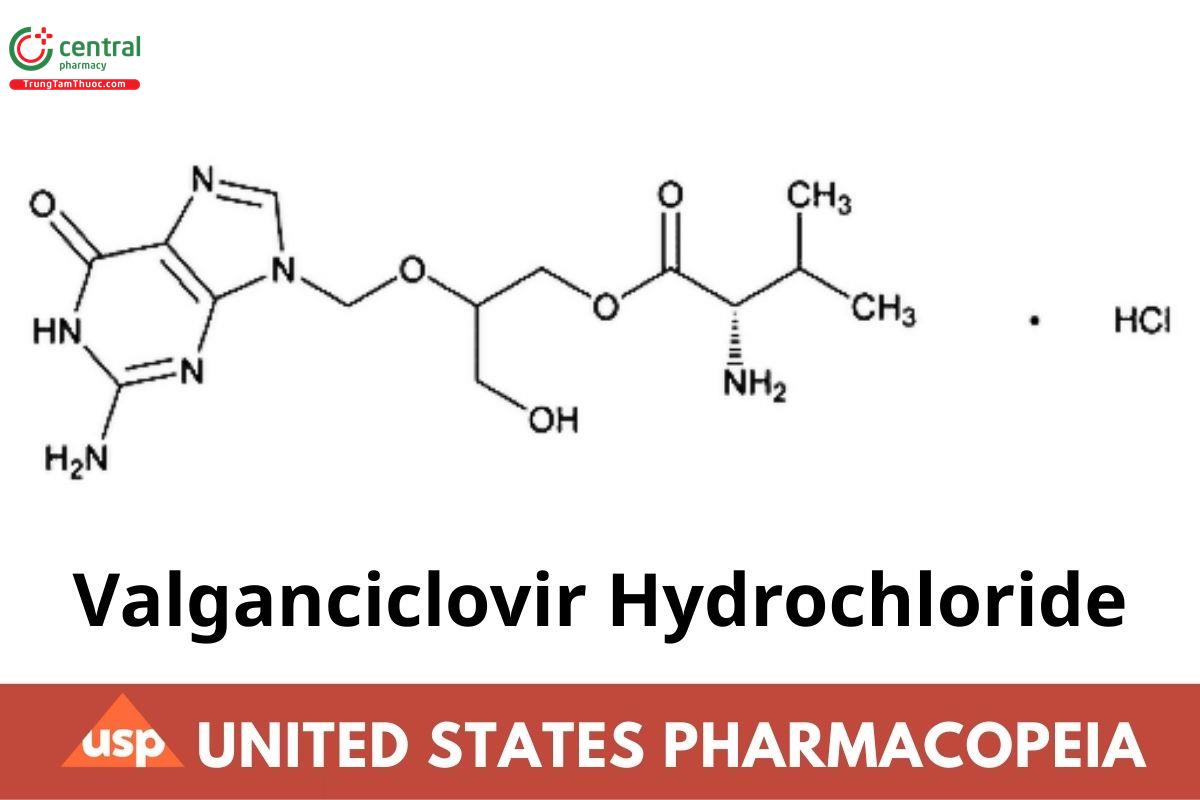
C14H22N6O5 · HCl 390.82
L-Valine, ester with 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine, monohydrochloride.
L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester, monohydrochloride CAS RN: 175865-59-5; UNII: 4P3T9QF9NZ.
1 DEFINITION
Valganciclovir Hydrochloride contains NLT 97.0% and NMT 102.0% of valganciclovir hydrochloride (C14H22N6O5 · HCl), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
B. The retention times of valganciclovir diastereomeric peaks of the Sample solution correspond to those of the Standard solution, as obtained in the Assay.
C. IDENTIFICATION TESTS-GENERAL (191), Chloride: Meets the requirements
3 ASSAY
PROCEDURE
Buffer: 11.5 g/L of monobasic ammonium phosphate in water. Adjust with phosphoric acid (85%) to a pH of 2.8 ± 0.2 prior to final dilution.
Mobile phase: Methanol and Buffer (8:92)
System suitability solution: 0.2 mg/mL of USP Valganciclovir Hydrochloride RS and 0.01 mg/mL of USP Methoxymethylguanine RS in 0.001 N hydrochloric acid
Standard solution: 0.2 mg/mL of USP Valganciclovir Hydrochloride RS in 0.001 N hydrochloric acid
Sample solution: 0.2 mg/mL of Valganciclovir Hydrochloride in 0.001 N hydrochloric acid
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 15-cm; 3.5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 20 µL
Run time: NLT 2.5 times the retention time of valganciclovir diastereomer peak 2
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for methoxymethylguanine and valganciclovir diastereomer peak 1 are 0.93 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 1.0 between methoxymethylguanine and valganciclovir diastereomer peak 1; NLT 3.0 between valganciclovir diastereomer peak 1 and valganciclovir diastereomer peak 2, System suitability solution
Tailing factor: NMT 2.0 for valganciclovir diastereomer peak 2, System suitability solution
Relative standard deviation: NMT 1.0% for the sum of valganciclovir diastereomer peak 1 and valganciclovir diastereomer peak 2, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of valganciclovir hydrochloride (C14H22N6O5 · HCl) in the portion of Valganciclovir Hydrochloride taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = sum of the peak responses of valganciclovir diastereomer peaks 1 and 2 from the Sample solution
rs = sum of the peak responses of valganciclovir diastereomer peaks 1 and 2 from the Standard solution
Cs = concentration of USP Valganciclovir Hydrochloride RS in the Standard solution (mg/mL)
Cu = concentration of Valganciclovir Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
RESIDUE ON IGNITION (281): NMT 0.10%
4.1 LIMIT OF ISOPROPYL ALCOHOL
Internal standard solution: Transfer 0.1 mL of 1.4-dioxane to a 100-mL volumetric flask and dilute with dimethylformamide to volume.
Standard stock solution: Transfer 1.0 mL of isopropyl alcohol and 0.1 mL of toluene to a 100-mL volumetric flask, and dilute with
dimethylformamide to volume. [NOTE-Toluene is used to verify the system suitability.]
Standard solution: Combine 2.0 mL of the Internal standard solution and 0.1 mL of the Standard stock solution.
Sample solution: Transfer 90-100 mg of Valganciclovir Hydrochloride to a vial, add 2.0 mL of the Internal standard solution, and mix.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.53-mm x 30-m capillary, coated with 3.0-pm film of phase G43
Carrier gas: Helium
Temperatures
Injection port: 250°
Detector: 300°
Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 40 | - | 40 | 10 |
| 40 | 25 | 240 | - |
| 240 | - | 240 | 15 |
Flow rate: 10.5 mL/min
Injection volume: 0.5 µL
Injection type: Split, split ratio 1:1
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 8 between 1,4-dioxane and toluene
Relative standard deviation: NMT 15% of the peak area ratio of isopropyl alcohol to 1,4-dioxane
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of isopropyl alcohol in the portion of Valganciclovir Hydrochloride taken:
Result = (Ru /Rs ) × (Cs /Cu ) × 100
Ru = peak area ratio of isopropyl alcohol to the internal standard from the Sample solution
Rs = peak area ratio of isopropyl alcohol to the internal standard from the Standard solution
Cs = concentration of isopropyl alcohol in the Standard solution (mg/mL)
Cu = concentration of the Sample solution (mg/mL)
Acceptance criteria: NMT 1.0%
Change to read:
Organic Impurities
Solution A: 11.5 g/L of monobasic ammonium phosphate in water. Adjust with phosphoric acid (85%) to a pH of 2.8 ± 0.2 prior to nal dilution.
Solution B: Methanol
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 92 | 8 |
| 5 | 92 | 8 |
| 15 | 80 | 20 |
| 30 | 30 | 70 |
| 30.1 | 92 | 8 |
| 45 | 92 | 8 |
System suitability solution: 0.2 mg/mL of USP Valganciclovir Hydrochloride RS and 0.01 mg/mL of USP Methoxymethylguanine RS in 0.001 N hydrochloric acid
Sample solution: 0.2 mg/mL of Valganciclovir Hydrochloride in 0.001 N hydrochloric acid
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Sample: System suitability solution
[Note— See Table 3 for the relative retention times.]
Suitability requirements
Resolution: NLT 1.0 between methoxymethylguanine and valganciclovir diastereomer peak 1; NLT 3.0 between valganciclovir diastereomer peak 1 and valganciclovir diastereomer peak 2
Tailing factor: NMT 2.0 for valganciclovir diastereomer peak 2
Analysis
Sample: Sample solution
Calculate the percentage of any individual impurity in the portion of Valganciclovir Hydrochloride taken:
Result = {(ri /Fi )/[rs + Σ(ri /Fi )]} × 100
ri = peak response for each individual impurity in the Sample solution
Fi = relative response factor (see Table 3)
rs = sum of the peak responses for valganciclovir diastereomers from the Sample solution
Acceptance criteria: See Table 3.
Table 3
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Guaninea | 0.28 | 1.9 | 0.25 |
| Ganciclovirb | 0.42 | 1.4 | 1.5 |
| Methoxymethylguanine | 0.81 | 1.0 | 0.3 |
| Valganciclovir diastereomer peak 1 | 0.86 | - | - |
| Valganciclovir diastereomer peak 2 | 1.0 | - | - |
| Isovalganciclovirc | 1.26 | 1.1 | 0.5 |
| Monoacetoxyganciclovird | 1.36 | 1.3 | 0.15 |
| Isomonochloroganciclovire | 1.47 | 1.3 | 0.1 |
| Monochloroganciclovirf | 1.52 | 1.4 | 0.1 |
| Bis-valine ester of ganciclovirg | 1.61 | 0.71 | 0.1 |
| Homolog of valganciclovirh | 1.66 | 1.0 | 0.25 |
| Ganciclovir monopropionatei | 2.09 | 1.1 | 0.15 |
| Valganciclovir dimer (stereoisomer A)j | 2.49 | 1.0 | 0.1 |
| Valganciclovir dimer (stereoisomer B)j | 2.52 | 1.0 | 0.1 |
| Valganciclovir dimer (stereoisomer C)j | 2.54 | 1.0 | 0.1 |
| Any individual unspecied impurity | - | 1.0 | 0.1 |
| Total impuritiesk | - | - | 3.0 |
a 2-Amino-1,9-dihydro-6H-purin-6-one.
b 2-Amino-9-{[(1,3-dihydroxypropan-2-yl)oxy]methyl}-1,9-dihydro-6H-purin-6-one.
c 3-[(2-Amino-6-oxo-1,6-dihydropurin-9-yl)methoxy]-2-hydroxypropyl L-valinate hydrochloride. (ERR 1-Sep-2023)
d 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]-3-hydroxypropyl acetate.
e 2-Amino-9-[(2-chloro-3-hydroxypropoxy)methyl]-1,9-dihydro-6H-purin-6-one.
f 2-Amino-9-{[(1-chloro-3- hydroxypropan-2-yl)oxy]methyl}-1,9-dihydro-6H-purin-6-one.
g 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]propane-1,3-diyl (2S,2'S)-bis(2-amino-3-methylbutanoate).
h 2-{[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]methoxy}-3-hydroxypropyl l-valinate hydrochloride.
i 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]-3-hydroxypropyl propionate.
j 2-{[2-({[(7-[({1-[(l-Valyl)oxy]-3-hydroxypropan-2-yl}oxy)methyl]-4-oxo-4,5,6,7-tetrahydro-3H-pyrrolo[2,3-d]pyrimidin-2-yl)amino]methyl}amino)-6-oxo-1,6-dihydro-9H-purin-9-yl]methoxy}-3-hydroxypropyl l-valinate dihydrochloride.
k Includes impurities from the tests for Organic Impurities and Limit of Ganciclovir Mono-N-methyl Valinate.
4.2 LIMIT OF GANCICLOVIR MONO-N-METHYL VALINATE
Solution A: 2.5 mL/L of triethylamine in water. Adjust with trifluoroacetic acid to a pH of 3 plus/minus 0.05
Solution B: Methanol
Mobile phase: See Table 4.
Table 4
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 93 | 7 |
| 10 | 93 | 7 |
| 20 | 70 | 30 |
| 20.1 | 93 | 7 |
| 35 | 93 | 7 |
Standard solution: 0.2 mg/mL of USP Valganciclovir Hydrochloride RS in 0.001 N hydrochloric acid
Sample solution: 0.2 mg/mL of Valganciclovir Hydrochloride in 0.001 N hydrochloric acid
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L11
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
[Note— The relative retention times for valganciclovir diastereomeric peak 2 and ganciclovir mono-N-methyl valinate are 1.0 and 1.2, respectively.]
Suitability requirements
Resolution: NLT 1.3 between valganciclovir diastereomeric peak 1 and valganciclovir diastereomeric peak 2
Tailing factor: NMT 1.5 for valganciclovir diastereomer peak 2
Analysis
Sample: Sample solution
Calculate the percentage of ganciclovir mono-N-methyl valinate in the portion of Valganciclovir Hydrochloride taken:
Result = (ru /rt ) × 100
ru = sum of the peak responses of ganciclovir mono-N-methyl valinate (diastereomers) from the Sample solution
rt = sum of all the peak responses from the Sample solution
Acceptance criteria: See Table 5.
Table 5
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Valganciclovir diastereomer peak 1 | 0.92 | - |
| Valganciclovir diastereomer peak 2 | 1.0 | - |
| Ganciclovir mono-N-methyl valinate diastereomer peak 1a,b | 1.1 | 0.3 |
| Ganciclovir mono-N-methyl valinate diastereomer peak 2a,b | 1.2 |
a 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]-3-hydroxypropyl methyl-l-valinate.
b Reported as the sum of diastereomers.
4.3 ENANTIOMERIC PURITY OF VALGANCICLOVIR
Mobile phase: 16.2 g/L of perchloric acid in water
System suitability solution: 0.2 mg/mL of USP Valganciclovir Hydrochloride RS and 0.02 mg/mL of USP D-Valganciclovir RS in 0.001 N hydrochloric acid
Sample solution: 0.2 mg/mL of Valganciclovir Hydrochloride in 0.001 N hydrochloric acid
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.0-mm × 15-cm; 5-µm packing L66
Temperature: Ambient
Flow rate: 0.8 mL/min
Injection volume: 20 µL
System suitability
Sample: System suitability solution
[NOTE-The typical retention times for the two D-valganciclovir diastereomer peaks and valganciclovir diastereomer peaks 1 and 2 are 8.9, 9.6, 13.2, and 14.7 min, respectively.]
Suitability requirements
Resolution: NLT 2.5 between the second peak of the D-valganciclovir pair and valganciclovir diastereomer peak 1
Analysis
Sample: Sample solution
Calculate the percentage of enantiomeric purity in the portion of Valganciclovir Hydrochloride taken:
Result = [rS /(rS + rIM )] × 100
rS = sum of the peak responses of valganciclovir diastereomer peaks 1 and 2 (R and S esters of l-valine) from the Sample solution
rIM = sum of the peak responses of the d-valganciclovir diastereomeric peaks (R and S esters of d-valine) from the Sample solution
Acceptance criteria: NLT 97.0%
5 SPECIFIC TESTS
Diastereomer Ratio
Analysis:
Using the chromatogram for the Sample solution in the test for Organic Impurities, calculate the percentage of valganciclovir diastereomers (R and S esters of l-valine):
Result = [rA /(rA + rB )] × 100
Result = [rB /(rA + rB )] × 100
rA = peak response for valganciclovir diastereomer peak 1
rB = peak response for valganciclovir diastereomer peak 2
Acceptance criteria: The diastereomer ratio is 45:55–55:45
Water Determination 〈921〉, Method I: NMT 8.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store at controlled room temperature.
USP Reference Standards 〈11〉
USP Methoxymethylguanine RS
2-Amino-9-(methoxymethyl)-1,9-dihydro-6H-purin-6-one.
C7H9N5O2 195.18
USP D-Valganciclovir RS
2-(RS)-[(2-Amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl d-valinate hydrochloride.
C14H22N6O5 · HCl 390.83
USP Valganciclovir Hydrochloride RS


