Valacyclovir Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
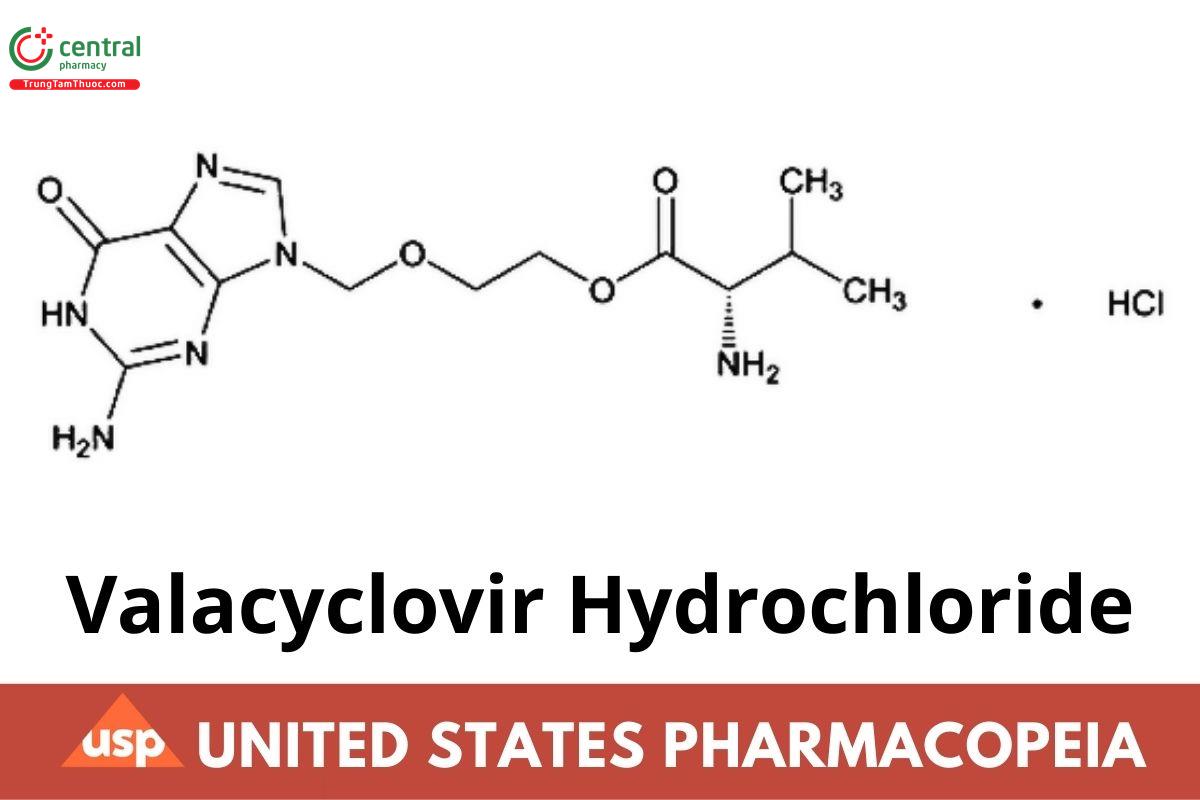
C13H20N6O4 · HCl 360.80
l-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy] ethyl ester, monohydrochloride;
l-Valine, ester with 9-[(2-hydroxyethoxy)methyl]guanine, monohydrochloride CAS RN: 124832-27-5; UNII: G447S0T1VC.
1 DEFINITION
Valacyclovir Hydrochloride contains NLT 95.0% and NMT 102.0% of valacyclovir hydrochloride (C13H20N6O4 · HCl), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
Change to read:
C. IDENTIFICATION TESTS GENERAL (191), Chemical Identification Tests. Chloride
Sample solution: 50 mg/mL of Valacyclovir Hydrochloride (USP 1-May-2023) in water
Acceptance criteria: Meets the requirements
ASSAY
Change to read:
PROCEDURE
Mobile phase: Methanol, water, and perchloric acid (5:95:0.5) (USP 1-May-2023)
Standard solution: 0.5 mg/mL of USP Valacyclovir Hydrochloride RS in 0.05 M hydrochloric acid. [NOTE-USP Valacyclovir Hydrochloride RS contains a detectable quantity of D-valacyclovir.]
Sample solution: 0.5 mg/mL of Valacyclovir Hydrochloride in 0.05 M hydrochloric acid
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4-mm x 15-cm; 5-µm packing L66
Column temperature: 10o
Flow rate: 0.75 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 2.0 between valacyclovir hydrochloride and D-valacyclovir
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of valacyclovir hydrochloride (C13H20N6O4 · HCl) in the portion of Valacyclovir Hydrochloride taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of valacyclovir from the Sample solution
rs = peak response of valacyclovir from the Standard solution
Cs = concentration of USP Valacyclovir Hydrochloride RS in the Standard solution (mg/mL)
Cu = concentration of Valacyclovir Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 95.0%–102.0% on the anhydrous and solvent-free basis
3 IMPURITIES
RESIDUE ON IGNITION (281)
Sample: 2 g
Acceptance criteria: NMT 0.1%
Delete the following:
LIMIT OF PALLADIUM (if present) (USP 1-May-2023)
Change to read:
ORGANIC IMPURITIES, PROCEDURE 1 (for related compounds E, F, and G)
Developing solvent: Methylene chloride, methanol, tetrahydrofuran, and ammonia solution (54:34:12:3)
Standard stock solution: Transfer 5 mg each of USP Valacyclovir Related Compound D RS and USP Valacyclovir Related Compound G RS, 10 mg of USP Valacyclovir Related Compound E RS, and 8.4 mg of USP Valacyclovir Related Compound F RS into a 10-ml volumetric flask. Add 2 mL of water with swirling, followed by 6 ml of alcohol, and sonicate for 20 min. Allow to cool, and dilute with alcohol to volume.
Standard solutions: Transfer 1.0 and 0.5 mL of the Standard stock solution into two separate 10-ml volumetric flasks. Dilute the solution in both flasks with alcohol to volume.
Sample solution: Transfer 250 mg of Valacyclovir Hydrochloride into a 5-mL volumetric flask. Add 2 mL of water, and sonicate for 20 min to dissolve. Add alcohol to about 95% of the flask volume. Cool, and dilute with alcohol to volume. Pass through a suitable filter of 0.45-µm pore size.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: TLC
Detector: UV, long and short wavelength
Plate: TLC plate coated with a 0.25-mm layer of chromatographic silica gel mixture. Prewash the plate with methanol.
Developing distance: NLT 7 cm from the origin
Application volume: 4 µL
Analysis
Samples: Standard solutions and Sample solution
Develop the plate to the specified distance. Remove the plate from the solvent chamber, and allow to dry. Examine the plate under short-wavelength UV light, and visually estimate the valacyclovir related compounds E and G in the sample using the appropriate standard spots. The chromatograms obtained with the Standard solutions each show three clearly separated spots due to valacyclovir related compounds D, E, and G. Spray the plate with 0.01% fluorescamine in ethylene dichloride, and examine the sprayed plate under long-wavelength UV light to estimate the level of valacyclovir related compound F in the sample using the appropriate standard spot. The relative Rf values and limits for each impurity are provided in Table 1.
Acceptance criteria: See Table 1
Table 1
| Name | Relative Rf Value | Acceptance Criteria, NMT (%) |
| Valacyclovir hydrochloride | 1 | - |
| Valacyclovir related compound Da | 1.1 | - |
| Valacyclovir related compound Eb | 1.3 | 0.2 |
| Valacyclovir related compound Fc | 1.8 | 0.1 |
| Valacyclovir related compound Gd | 1.9 | 0.05 |
a This impurity is quantitated using Procedure 2.
b 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl N-benzyloxycarbonyl-l-valinate. (USP 1-May-2023)
c 2-Hydroxyethyl valinate 4-methylbenzenesulfonate.(USP 1-May-2023)
d N,N-Dimethylpyridin-4-amine.
Change to read:
Organic Impurities, Procedure 2
Solution A: 0.3% w/w triuoroacetic acid solution in water
Solution B: 0.3% w/w triuoroacetic acid solution in methanol
Diluent: Alcohol and water(20:80) (USP 1-May-2023)
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 90 | 10 |
| 5 | 90 | 10 |
| 35 | 60 | 40 |
| 35.01 | 90 | 10 |
| 45 | 90 | 10 |
System suitability solution: 0.4 mg/mL of USP Valacyclovir Hydrochloride RS, 0.8 µg/mL of USP Valacyclovir Related Compound C RS, and 1.6 µg/mL of USP Acyclovir Related Compound A RS in Diluent
Sample solution: 0.4 mg/mL of Valacyclovir Hydrochloride in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 25-cm; 5-µm packing L11
Column temperature: 15°
Flow rate: 0.8 mL/min
Injection volume: 10 µL
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 1.5 between valacyclovir and valacyclovir related compound C; NLT 1.5 between valacyclovir related compound C and acyclovir related compound A
Tailing factor: NMT 1.5 for the valacyclovir hydrochloride peak
Analysis
Sample: Sample solution
Calculate the percentage of each individual impurity in the portion of Valacyclovir Hydrochloride taken:
Result = (ru /rt ) × 100
ru = peak response of any impurity in the Sample solution
rt = sum of all the peak responses from the Sample solution
Acceptance criteria: See Table 3.
Table 3
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Guanine (near solvent front)a (USP 1-May2023) | 0.31 | - |
| Acyclovira,b | 0.42 | - |
| Acyclovir alaninatec | 0.54 | 0.2 |
| Valacyclovir | 1.00 | - |
| Valacyclovir related compound Cd | 1.06 | 0.3 |
| Acyclovir related compound Aa,e | 1.09 | - |
| Valacyclovir related compound Df | 1.17 | 0.5 |
| Acyclovir isoleucinateg | 1.30 | 0.2 |
| N-Formyl valacyclovirh | 1.61 | 0.8 |
| Guaninyl valacycloviri | 1.66 | 0.2 |
| Bis valacyclovirj | 2.0 | 0.3 |
| Any unspecied impurity | - | 0.1 |
a This impurity is quantitated by the Procedure 3 method.
b 9-[(2-Hydroxyethoxy)methyl]guanine. (USP 1-May-2023)
c 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl l-alaninate. (USP 1-May-2023)
d 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl N-methyl-l-valinate.
e 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl acetate.
f 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl N-ethyl-l-valinate.
g 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl l-isoleucinate. (USP 1-May-2023)
h 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl N-formyl-l-valinate.(USP 1-May-2023)
i 2-[(6-Oxo-2-{[(6-oxo-6,9-dihydro-1H-purin-2-yl)aminomethyl]amino}-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl l-valinate. (USP 1-May-2023)
j {Methylenebis[azanediyl(6-oxo-1,6-dihydro-9H-purine-2,9-diyl)methyleneoxyethan-2,1-diyl]} di-l-valinate. (USP 1-May-2023)
Change to read:
Organic Impurities, Procedure 3
Mobile phase, Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each individual impurity in the portion of Valacyclovir Hydrochloride taken:
Result = (ru /rs ) × (Cs /Cu ) × (1/F) × 100
ru = peak response of guanine plus acyclovir or acyclovir acetate or d-valacyclovir from the Sample solution
rs = peak response of valacyclovir from the Standard solution
Cs = concentration of USP Valacyclovir Hydrochloride RS in the Standard solution (mg/mL)
Cu = concentration of Valacyclovir Hydrochloride in the Sample solution (mg/mL)
F = relative response factor (see Table 4)
Acceptance criteria: See Table 4.
Table 4
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Guanine and acyclovir (USP 1-May-2023)a | 0.18 | 1.51 | 2.0 |
| Acyclovir related compound Ab | 0.42 | 1.12 | 0.2 |
| D-Valacyclovirc | 0.55 | 1.0 | 3.0 |
| Valacyclovir | 1.0 | - | - |
a 9-[(2-Hydroxyethoxy)methyl]guanine. (USP 1-May-2023)
b 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl acetate.
c 2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl d-valinate. (USP 1-May-2023)
Total organic impurities: NMT 5.0% for the sum of all impurities from Organic Impurities, Procedures 1, 2, and 3
4 SPECIFIC TESTS
Change to read:
Water Determination 〈921〉, Method I: For the anhydrous form: NMT 2.0% (200 mg of sample); if labeled as the hydrous form: 3.0% (USP 1-May-2023) –11.0%
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and store at a temperature below 30°.
Labeling: Where it is the hydrous form, the label so indicates.
Change to read:
USP Reference Standards 〈11〉
USP Acyclovir Related Compound A RS
[Note—USP Acyclovir Related Compound A AS is equivalent.]
2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl acetate.
C10H13N5O4 267.25 (USP 1-May-2023)
USP Valacyclovir Hydrochloride RS
USP Valacyclovir Related Compound C RS
2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl N-methyl-l-valinate hydrochloride.
C14H22N6O4 · HCl 374.83 (USP 1-May-2023)
USP Valacyclovir Related Compound D RS
2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9- (USP 1-May-2023) yl)methoxy]ethyl N-ethyl-l-valinate.
C15H24N6O4 352.40 (USP 1-May-2023)
USP Valacyclovir Related Compound E RS
2-[(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)methoxy]ethyl N-benzyloxycarbonyl-l-valinate. (USP 1-May-2023)
C21H26N6O6 458.48 (USP 1-May-2023)
USP Valacyclovir Related Compound F RS
2-Hydroxyethyl valinate 4-methylbenzenesulfonate. (USP 1-May-2023)
C7H15NO3 · C7H8O3S 333.40
USP Valacyclovir Related Compound G RS
N,N-Dimethylpyridin-4-amine.
C7H10N2 122.17


