Trimethobenzamide Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
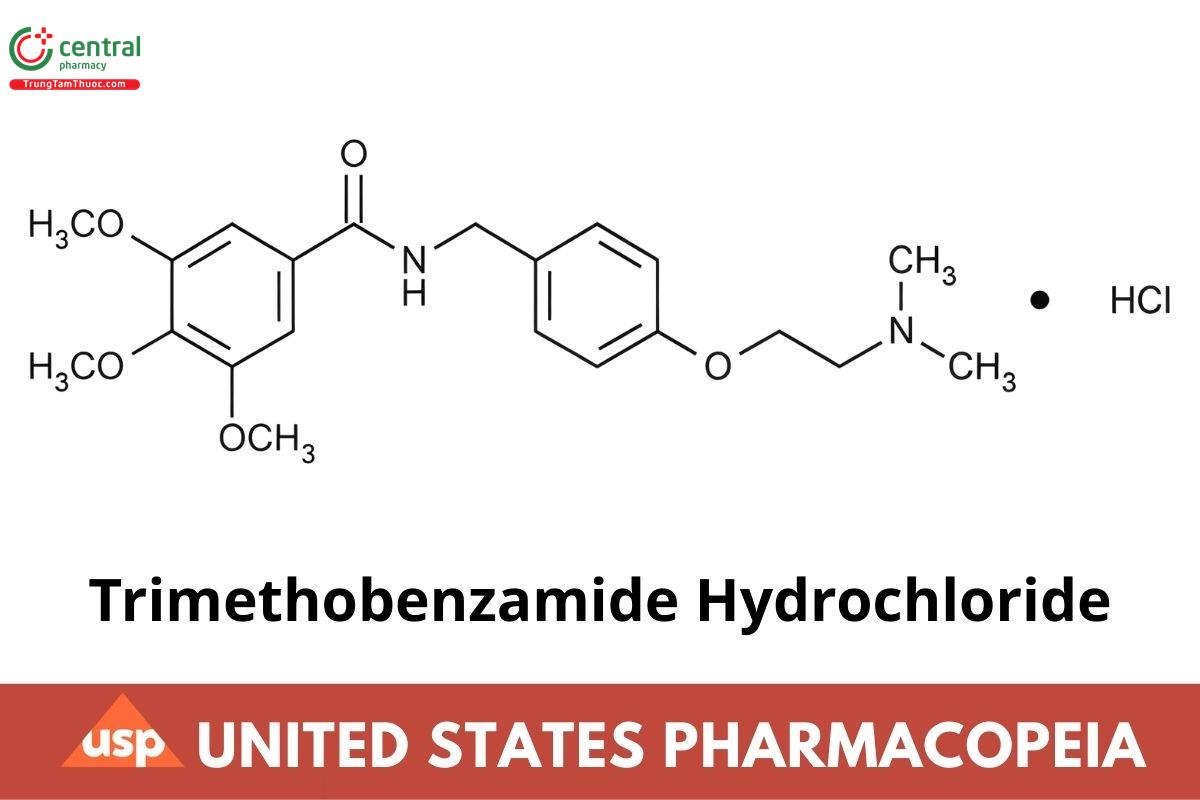
C21H28N2O5 · HCI 424.92
Benzamide, N-[[4-[2-(dimethylamino) ethoxy]phenyl]methyl]-3,4,5-trimethoxy-, monohydrochloride.
▲N-[4-[2-(Dimethylamino) ethoxylbenzyl]-3,4,5-trimethoxybenzamide monohydrochloride ▲(ERR 1-Aug-2023) CAS RN®: 554-92-7; UNII: WDQ5P1SX7Q.
>> Trimethobenzamide Hydrochloride, dried at 105° for 4 hours, contains not less than 98.5 percent and not more than 100.5 percent of C21H28N2O5 · HCI.
1 Packaging and storage-
Preserve in well-closed containers.
2 USP REFERENCE STANDARDS (11)-
USP Trimethobenzamide Hydrochloride RS
2.1 Identification-
A: Spectroscopic Identification Tests (197), Infrared Spectroscopy : 197K.
B: Spectroscopic Identification Tests (197). Ultraviolet-Visible Spectroscopy: 1970-
Solution: 20 µg per mL.
Medium: 0.1 N hydrochloric acid.
Absorptivities at 258 nm, calculated on the dried basis, do not differ by more than 3.0%.
C: It meets the requirements of the Thin-layer Chromatographic Identification Test (201). Prepare the test solution by dissolving 10 mg of Trimethobenzamide Hydrochloride in 10.0 mL of methanol. Apply 10-µL portions of the test solution and the Standard solution to the plate, and develop in a solvent system consisting of a mixture of ethyl acetate, alcohol, and ammonium hydroxide (90:10:5).
D: It meets the requirements of the tests for Chloride (191).
MELTING RANGE, Class / (741): between 186 and 190°.
LOSS ON DRYING (731)-Dry it at 105° for 4 hours: it loses not more than 0.5% of its weight.
RESIDUE ON IGNITION (281): not more than 0.1%.
2.2 Assay-
Dissolve about 1.3 g of Trimethobenzamide Hydrochloride, previously dried and accurately weighed, in 80 mL of glacial acetic acid and 15 mL of mercuric acetate TS. Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically using suitable electrodes. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 42.49 mg of C21H28N2O5 · HCI


