Triethyl Citrate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
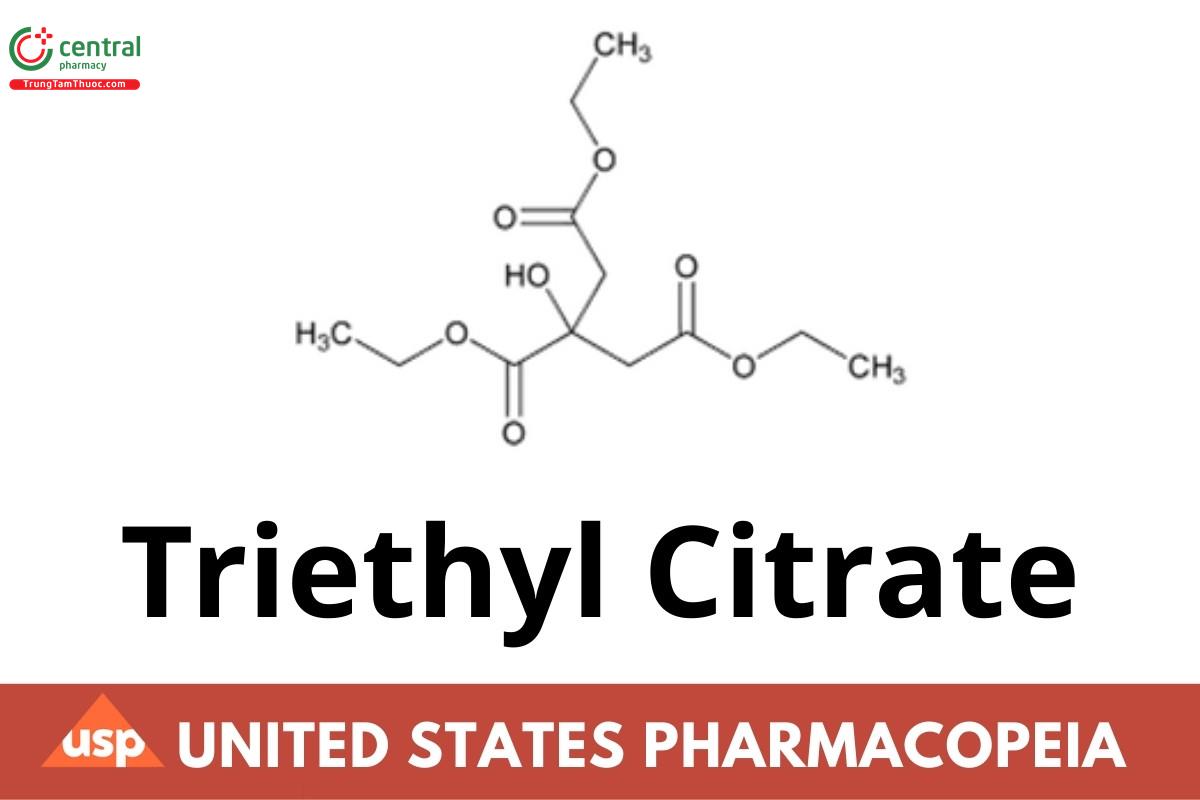
Triethyl Citrate contains NLT 97.0% and NMT 102.0% (NF 1-May-2023) of triethyl citrate (C12H20O7), calculated on the anhydrous basis.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS 〈197〉 , Infrared Spectroscopy: 197F Change to read:
B. CHROMATOGRAPHIC IDENTITY: (NF 1-M -2023) The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, (NF 1-May-2023) as obtained in the Assay.
3 ASSAY
Change to read:
PROCEDURE
Diluent: Methylene chloride
Internal standard solution: 1 mg/mL of trimethyl citrate in Diluent
Standard solution: 1 mg/mL of USP Triethyl Citrate RS in Internal standard solution Sample solution: 1 mg/mL of Triethyl Citrate in Internal standard solution
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 30-m; 0.5-µm layer of phase G42
Temperatures
Injector: 225°
Detector: 275° Column: See Table 1.
Table 1
Carrier gas: Helium Flow rate: 2.3 mL/min
Injection volume: 1 µL
Injection type: Split, split ratio 30:1
System suitability
Sample: Standard solution
[NOTE—The retention time for triethyl citrate is about 14.6 min. The relative retention times for trimethyl citrate and triethyl citrate are 0.9 and 1.0, respectively.]
Suitability requirements
Tailing factor: NMT 1.5 for the triethyl citrate and (ERR 1-May-2023) trimethyl citrate peaks Relative standard deviation: NMT 1.0% of the peak area ratio of triethyl citrate to trimethyl citrate
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of triethyl citrate (C12H20O7) in the portion of Triethyl Citrate taken:
Result = (RU/RS) × (CS/CU) × 100
RU = peak area ratio of triethyl citrate to trimethyl citrate from the Sample solution
RS = peak area ratio of triethyl citrate to trimethyl citrate from the Standard solution
CS = concentration of USP Triethyl Citrate RS in the Standard solution (mg/mL)
CU = concentration of Triethyl Citrate in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–102.0% on the anhydrous basis (NF 1-May-2023)
Add the following:
4 IMPURITIES
ORGANIC IMPURITIES
Diluent and Chromatographic system: Proceed as directed in the Assay. Standard solution A: 0.02 mg/mL of USP Triethyl Aconitate RS in Diluent Standard solution B: 0.02 mg/mL of USP Triethyl Citrate RS in Diluent Sample solution: 10 mg/mL of Triethyl Citrate in Diluent
System suitability
Samples: Standard solution A and Standard solution B Suitability requirements
Relative standard deviation: NMT 3.0% for the triethyl aconitate peak, Standard solution A; NMT 3.0% for the triethyl citrate peak, Standard solution B
Analysis
Samples: Standard solution A, Standard solution B, and Sample solution [NOTE—See Table 2 for the relative retention times.]
Table 2
a Triethyl (E)-prop-1-ene-1,2,3-tricarboxylate.
Calculate the percentage of triethyl aconitate in the portion of Triethyl Citrate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak area of triethyl aconitate from the Sample solution
rS = peak area of triethyl aconitate from Standard solution A
CS = concentration of USP Triethyl Aconitate RS in Standard solution A (mg/mL)
CU = concentration of Triethyl Citrate in the Sample solution (mg/mL)
Calculate the percentage of each individual impurity, excluding any solvent peaks, in the portion of Triethyl Citrate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak area of each individual impurity from the Sample solution
rS = peak area of triethyl citrate from Standard solution B
CS = concentration of USP Triethyl Citrate RS in Standard solution B (mg/mL)
CU = concentration of Triethyl Citrate in the Sample solution (mg/mL)
Acceptance criteria
Triethyl aconitate: NMT 0.2% Individual impurity: NMT 0.2%
Total impurities: NMT 0.5% (NF 1-May-2023)
5 SPECIFIC TESTS
Delete the following:
SPECIFIC GRAVITY 〈841〉 (NF 1-May-2023)
Delete the following:
REFRACTIVE INDEX 〈831〉 (NF 1-May-2023)
ACIDITY
Neutralized isopropyl alcohol: To a suitable quantity of isopropyl alcohol add 2–3 drops of bromothymol blue TS and just sufficient 0.10 N sodium hydroxide dropwise to produce a faint blue color. [NOTE—Prepare Neutralized isopropyl alcohol just prior to use.]
Sample solution: 32.0 g of Triethyl Citrate in 30 mL of Neutralized isopropyl alcohol
Analysis: Add bromothymol blue TS, and titrate with 0.10 N sodium hydroxide to a faint blue endpoint. Acceptance criteria: NMT 1.0 mL of 0.10 N sodium hydroxide is required.
WATER DETERMINATION 〈921〉 , Method I: NMT 0.25%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.
Change to read:
USP REFERENCE STANDARDS 〈11〉
USP Triethyl Aconitate RS (NF 1-May-2023)
USP Triethyl Citrate RS


