Triclosan
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
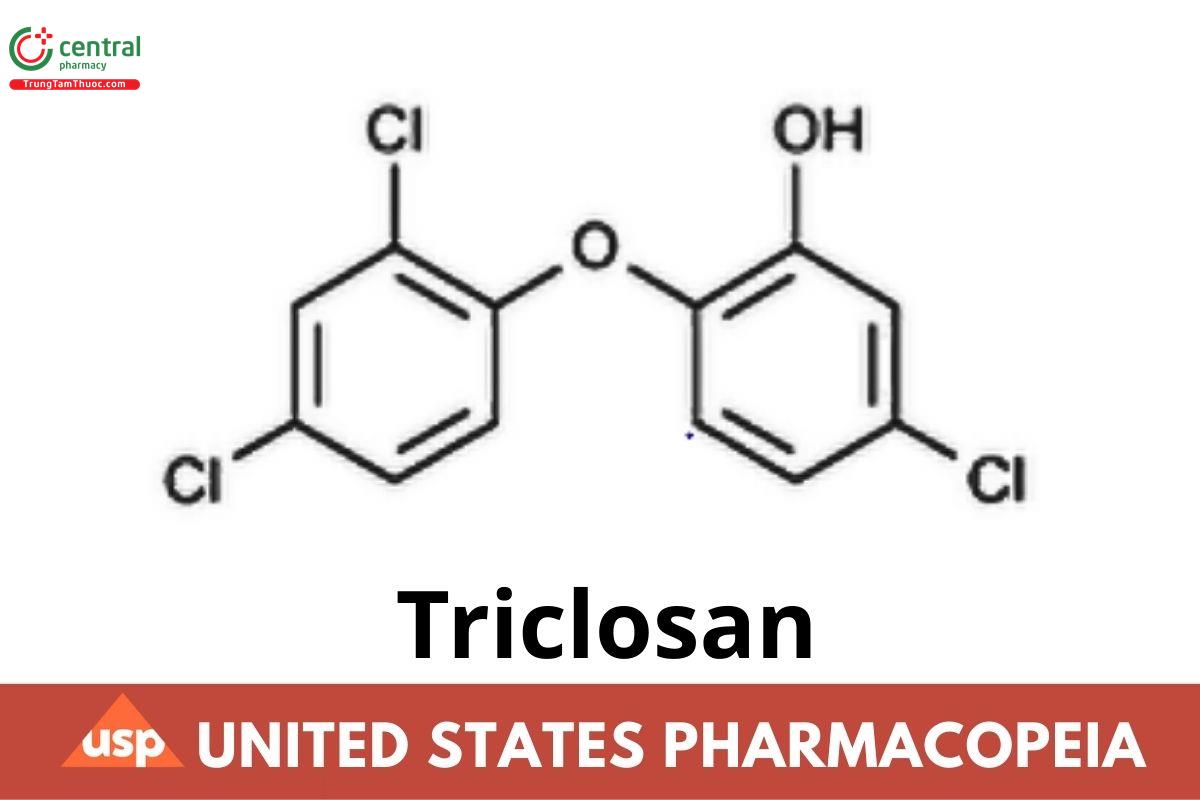
C₁₂H₇Cl₃O₂ 289.54
Phenol, 5-chloro-2-(2,4-dichlorophenoxy)-;
2,4,4′-Trichloro-2′-hydroxydiphenyl ether CAS RN®: 3380-34-5; UNII: 4NM5039Y5X.
1 DEFINITION
Triclosan contains NLT 97.0% and NMT 103.0% of triclosan (C₁₂H₇Cl₃O₂), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K or 197A
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Change to read:
3.1 Procedure
Standard solution: 0.4 mg/mL of USP Triclosan RS in ethyl acetate
Sample solution: 0.4 mg/mL of Triclosan in ethyl acetate
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: GC
- Detector: Flame ionization
- Column: 0.53-mm × 15-m; coated with a 1.0-µm film of phase G3
- Temperatures
- Injection port: 200°
- Detector: 260°
- Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 34 | 20 | 140 | 0 |
| 140 | 4 | 240 | NLT 5 |
- Carrier gas: Helium
- Pressure: 6 psi
- Injection volume: 2 µL
System suitability
- Sample: Standard solution
- Suitability requirements
- Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of triclosan (C₁₂H₇Cl₃O₂) in the portion of Triclosan taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of triclosan from the Sample solution
rₛ = peak response of triclosan from the Standard solution
Cₛ = concentration of USP Triclosan RS in the Standard solution (mg/mL)
Cᵤ = concentration of Triclosan in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–103.0% on the anhydrous basis
4 IMPURITIES
4.1 Organic Impurities
Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
System suitability
- Sample: Standard solution
- Suitability requirements
- Relative standard deviation: NMT 2.0%
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Triclosan taken:
Result = (rU/rT) × 100
rU = peak response of each impurity in the Sample solution
rT = sum of all peak responses in the Sample solution
Acceptance criteria
- Individual impurities: NMT 0.1%
- Total impurities: NMT 0.5%
Change to read:
4.2 Limit of 1,3,7-Trichlorodibenzo-p-dioxin, 2,8-Dichlorodibenzo-p-dioxin, 2,8-Dichlorodibenzofuran, and 2,4,8-Trichlorodibenzofuran
Mobile phase: Acetonitrile, water, and glacial acetic acid (70:30:0.1)
Standard solution: Use USP Triclosan Related Compounds Mixture A RS
Sample solution: Transfer 2.0 g of Triclosan into a screw-capped centrifuge tube. Add 5 mL of 2 N potassium hydroxide, and shake for 10 min to dissolve. Add 3 mL of n-hexane, shake for 10 min, and allow the phases to separate. Transfer the organic layer to a suitable container, add another 3 mL of n-hexane to the aqueous layer, shake for 10 min, and allow the phases to separate. Transfer the organic layer to the previous extract, discard the aqueous layer, add 3 mL of 2 N potassium hydroxide to the combined organic layers, shake for 10 min, and allow the phases to separate. Discard the aqueous layer, add another 3 mL of 2 N potassium hydroxide to the combined organic layers, shake for 10 min, and allow the phases to separate. Transfer the organic layer to a suitable container, and evaporate with the aid of a stream of nitrogen to dryness. Dissolve the residue in 1.0 mL of methanol.
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 220 nm
- Column: 4.6-mm × 25-cm; 5-μm packing L1
- Flow rate: 1.5 mL/min
- Injection volume: 20 µL
System suitability
- Sample: Standard solution
- Suitability requirements
- Relative standard deviation: NMT 15.0% from the 2,8-dichlorodibenzo-p-dioxin peak
Analysis
Samples: Standard solution and Sample solution
Calculate the concentration of each impurity in the portion of Triclosan taken:
Result = (rᵤ/rₛ) × (Cₛ/W) × V
rᵤ = peak response of each impurity from the Sample solution
rₛ = peak response of the corresponding impurity in USP Triclosan Related Compounds Mixture A RS from the Standard solution
Cₛ = concentration of the corresponding impurity in USP Triclosan Related Compounds Mixture A RS in the Standard solution (µg/mL)
W = weight of Triclosan taken to prepare the Sample solution (g)
V = volume of the Sample solution, 1 mL
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (ppm) |
| 2,8-Dichlorodibenzofuran | 0.59 | 0.25 |
| 2,8-Dichlorodibenzo-p-dioxin | 0.71 | 0.5 |
| 2,4,8-Trichlorodibenzofuran | 0.88 | 0.5 |
| 1,3,7-Trichlorodibenzo-p-dioxin | 1.00 | 0.25 |
Change to read:
Limit of 2,3,7,8-Tetrachlorodibenzo-p-dioxin and 2,3,7,8-Tetrachlorodibenzofuran
[Caution-2,3,7,8-Tetrachlorodibenzo-p-dioxin and 2,3,7,8-tetrachlorodibenzofuran are extremely toxic substances. Exercise all necessary precautions in the conduct of this procedure.]
Stationary phase A: Transfer 10 g of silica gel to a suitable container, add 3 mL of 1 N sodium hydroxide, and mix.
Stationary phase B: Transfer 60 g of silica gel to a suitable container, add 74 mL of concentrated sulfuric acid, and mix.
Chromatographic column A: Transfer 5.1 g of Stationary phase A, 0.5 g of silica gel, 6.2 g of Stationary phase B, and 3.2 g of sodium sulfate to a glass chromatographic column having an internal diameter of 10 mm. Wash the column with 50 mL of n-hexane, and discard the eluate.
Chromatographic column B: Transfer 2.5 g of alumina and 2.5 g of sodium sulfate to a glass chromatographic column having an internal diameter of 6 mm. Wash the column with 30 mL of n-hexane, and discard the eluate.
Internal standard solution: 1.0 pg/µL each of ¹³C-labeled 2,3,7,8-tetrachlorodibenzo-p-dioxin and ¹³C-labeled 2,3,7,8-tetrachlorodibenzofuran prepared as follows. Transfer quantities of ¹³C-labeled 2,3,7,8-tetrachlorodibenzo-p-dioxin and ¹³C-labeled 2,3,7,8-tetrachlorodibenzofuran, in nonane, and dilute if necessary, with 2,2,4-trimethylpentane.
Sample solution: Transfer 30 g of Triclosan into a separatory funnel. Add 30 µL of Internal standard solution, and dissolve in 200 mL of 1 N sodium hydroxide. Extract with four 30-mL portions of n-hexane and combine the extracts. Wash the combined extracts with 20 mL of water, extract the washing with 15 mL of n-hexane, and add the extract to the other combined extracts. Add 3 g of anhydrous sodium sulfate to the combined extracts, allow to stand for 30 min, quantitatively transfer to an appropriate round-bottom flask, and distill, using a distillation apparatus with a Vigreux column, until about 1 mL remains. Transfer this solution to the top of Chromatographic column A, and elute with 50 mL of n-hexane. Collect the eluate on top of Chromatographic column B, and elute with 30 mL of a mixture of n-hexane and methylene chloride (98:2), discarding the eluate. Elute with 40 mL of a mixture of n-hexane and methylene chloride (50:50), collecting the eluates in a round-bottom flask. Distill the combined eluates, using a distillation apparatus with a Vigreux column, until 1 mL remains.
Further concentrate this solution with the aid of a stream of nitrogen to 50 µL, evaporate at room temperature to dryness, and dissolve in 10 µL of 2,2,4-trimethylpentane.
Chromatographic system
- (See Chromatography 〈621〉, System Suitability and Mass Spectrometry 〈736〉.)
- Mode: GC
- Detector: High-resolution mass spectrometer with an electron-impact ionization source
- Column: 0.25-mm × 60-m; coated with a 0.15-µm film of phase G48
- Temperatures
- Injection port: 280°
- Column: See Table 3.
Table 3
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 80 | 0 | 80 | 1 |
| 80 | 20 | 220 | 0 |
| 220 | 2 | 270 | NLT 20 |
- Carrier gas: Helium
- Injection volume: 1 µL
System suitability
- Sample: Internal standard solution
- Suitability requirements
- Signal-to-noise ratio: NLT 50 at a mass-to-charge (m/z) ratio of 321.89
Analysis
Sample: Sample solution
Measure the peak responses at m/z ratios of 319.90, 321.89, 331.88, 333.93, 303.90, 305.90, 315.94, and 317.94.
Acceptance criteria: The peak response for 2,3,7,8-tetrachlorodibenzo-p-dioxin at an m/z ratio of 319.90 is NMT the peak response of the associated internal standard at an m/z ratio of 331.88; the peak response for 2,3,7,8-tetrachlorodibenzofuran at an m/z ratio of 303.90 is NMT the peak response of the associated internal standard at an m/z ratio of 315.94.
Change to read:
4.3 Limit of Monochlorophenols and 2,4-Dichlorophenol
Buffer: Dissolve 1.38 g of sodium phosphate, monobasic, anhydrous and 1.42 g of sodium phosphate, dibasic in 1 L of water.
Mobile phase: Acetonitrile and Buffer (50:50)
Standard solution: 0.5 µg/mL of USP Parachlorophenol RS and 0.1 µg/mL of USP 2,4-Dichlorophenol RS prepared as follows. Dissolve in acetonitrile a suitable amount each of USP Parachlorophenol RS and USP 2,4-Dichlorophenol RS in a suitable volumetric flask, and add an equal volume of water. Dilute appropriately a portion of this solution with a mixture of acetonitrile and water (50:50).
Sample solution: Transfer 250 mg of Triclosan into a 25-mL, low-actinic volumetric flask. Dissolve in 20 mL of acetonitrile, and dilute with water to volume.
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: Coulometric electrochemical with electrode 1 set at 0.45 V and electrode 2 set at 0.75 V, both having a positive (oxidative) polarity
- Column: 4.6-mm × 25-cm; 5-μm packing L1
- Flow rate: 1 mL/min
- Injection volume: 20 µL
System suitability
- Sample: Standard solution
- Suitability requirements
- Relative standard deviation: NMT 9.0% for 2,4-dichlorophenol
Analysis
Samples: Standard solution and Sample solution
[Note-The relative retention times for parachlorophenol and 2,4-dichlorophenol are 0.7 and 1.0, respectively.]
Acceptance criteria: The peak responses for parachlorophenol and 2,4-dichlorophenol of the Sample solution are NMT the corresponding peaks of the Standard solution.
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I: NMT 0.1%
Completeness of Solution 〈641〉
Sample: 1.40 g/10 mL of acetone
Acceptance criteria: Meets the requirements
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
Change to read:
USP Reference Standards 〈11〉
USP 2,4-Dichlorophenol RS
2,4-Dichlorophenol.
C₆H₄Cl₂O 163.00
USP Parachlorophenol RS
4-Chlorophenol.
C₆H₅ClO 128.56
USP Triclosan RS
USP Triclosan Related Compounds Mixture A RS
Contains a mixture of the following four compounds:
2,8-Dichlorodibenzofuran.
2,4,8-Trichlorodibenzofuran.
2,8-Dichlorodibenzodioxin.
1,3,7-Trichlorodibenzodioxin.


