Triclocarban
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
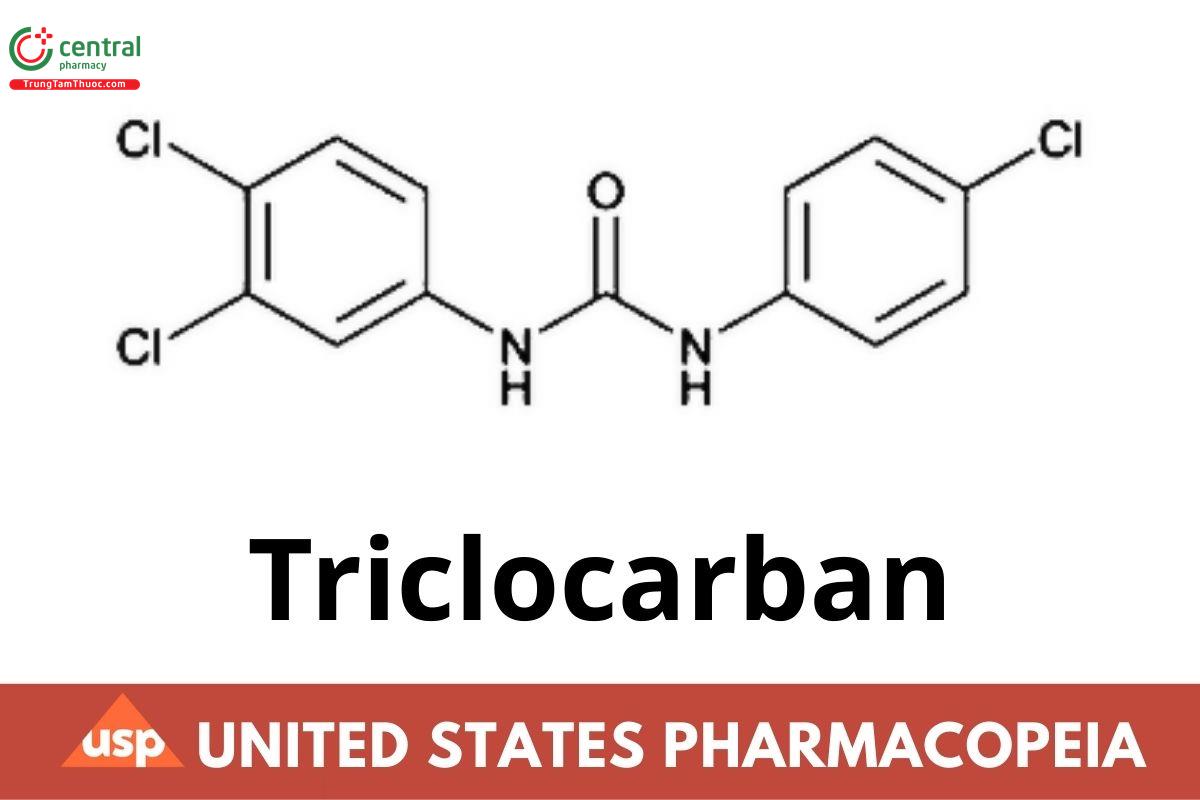
C13H9Cl3N2O 315.58
Urea, N-(4-chlorophenyl)-N'-(3,4-dichlorophenyl)-; 3,4,4'-Trichlorocarbanilide; 1-(4-Chlorophenyl)-3-(3,4-dichlorophenyl)urea CAS RN: 101-20-2; UNII: BGG1Y1ED0Y.
1 DEFINITION
Triclocarban contains NLT 97.0% and NMT 103.0% of triclocarban (C₁₂H₂CIN₂O), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Mobile phase: Acetonitrile, methanol, water, and glacial acetic acid (400:500:300:1.5)
System suitability solution: 0.1 mg/mL each of USP Triclocarban RS, USP Triclocarban Related Compound A RS, and USP Triclocarban Related Compound C RS in acetonitrile
Standard solution: 0.1 mg/mL of USP Triclocarban RS in acetonitrile. The solution is stable for 12 h.
Sample solution: 0.1 mg/mL of Triclocarban in acetonitrile. The solution is stable for 12 h.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 265 nm
Column: 4.6-mm x 25-cm; 5-µm packing L1
Flow rate: 2 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
[NOTE-The relative retention times for triclocarban related compound A, triclocarban, and triclocarban related compound C are 0.7, 1.0, and 1.6, respectively.]
Suitability requirements
Resolution: NLT 2.0 between triclocarban related compound A and triclocarban, NLT 2.0 between triclocarban and triclocarban related compound C, System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of triclocarban (C13H9Cl3N2O) in the portion of Triclocarban taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Triclocarban RS in the Standard solution (mg/mL)
Cu = concentration of Triclocarban in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–103.0% on the dried basis
4 IMPURITIES
4.1 ORGANIC IMPURITIES
Mobile phase: Acetonitrile, methanol, water, and glacial acetic acid (370:380:250:1)
Internal standard solution: 0.12 mg/mL of p-nitrochlorobenzene in methanol
Standard solution: 2 µg/mL each of USP Triclocarban RS. USP Triclocarban Related Compound A RS, USP Triclocarban Related Compound C RS, USP Triclocarban Related Compound D Mixture RS, and 2.4 µg/mL of p-nitrochlorobenzene from the Internal standard solution. Dissolve and dilute with methanol to volume.
Sample solution: 0.2 mg/mL of Triclocarban and 2.4 µg/ml. of p-nitrochlorobenzene from the Internal standard solution. Dissolve and dilute with methanol to volume.
Chromatographic system
(See Chromatography (621). System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 15-cm; 5-µm packing L1
Column temperature: 40o
Flow rate: 1.3 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Signal-to-noise ratio: NLT 10 for triclocarban related compound A, triclocarban related compound C, and triclocarban related compound D mixture
Relative standard deviation: NMT 2.0% for triclocarban
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Triclocarban taken:
Result = (Ru /Rs ) × (Cs /Cu ) × 100
Ru = peak response ratio of each impurity to the internal standard from the Sample solution
Rs = peak response ratio of corresponding specied impurity to the internal standard from the Standard solution (use the triclocarban peak response ratio to calculate for any individual impurity)
Cs = concentration of each impurity in the Standard solution (µg/mL)
Cu = concentration of Triclocarban in the Sample solution (µg/mL)
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| p-Nitrochlorobenzenea | 0.45 | - |
| Triclocarban related compound Ab | 0.64 | 1.0 |
| Triclocarban | 1.00 | - |
| Triclocarban related compound Cc | 1.66 | 1.0 |
| Triclocarban related compound D mixtured | 2.72 and 3.25 | 1.0 |
| Any other individual impurity | - | 1.0 |
| Total impurities | - | 2.0 |
a Internal standard—for information only.
b 1,3-Bis(4-chlorophenyl)urea.
c 1,3-Bis(3,4-dichlorophenyl)urea.
d Consists of two components, triarylbiuret 1 and triarylbiuret 2. Triarylbiuret 1 is [3,5-bis(4-chlorophenyl)-1-(3,4-dichlorophenyl)biuret] with RRT of 2.72. Triarylbiuret 2 is [1,5-bis(4-chlorophenyl)-3-(3,4-dichlorophenyl)biuret] with RRT of 3.25. The acceptance criteria is the sum of triarylbiuret 1 and triarylbiuret 2.
4.2 CHLOROANILINES
Dimethylformamide solution: 5% dimethylformamide in 1 N hydrochloric acid
Sodium nitrite solution: 2.5 mg/mL of sodium nitrite in water
Ammonium sulfamate solution: 25 mg/mL of ammonium sulfamate in water
N-(1-naphthyl)ethylenediamine dihydrochloride solution: 10 mg/mL in water
Standard stock solution 1: 560 µg/mL of 3,4-dichloroaniline and 440 µg/mL of p-chloroaniline dissolved in 2% of the nal volume with the Dimethylformamide solution. Dilute with 0.2 N hydrochloric acid to volume.
Standard stock solution 2: 56 µg/mL of 3,4-dichloroaniline and 44 µg/mL of p-chloroaniline in 0.2 N hydrochloric acid from Standard stock solution 1
Standard stock solution 3: 5.6 µg/mL of 3,4-dichloroaniline and 4.4 µg/mL of p-chloroaniline in 0.2 N hydrochloric acid from Standard stock solution 2
Standard stock solution 4: 0.56 µg/mL of 3,4-dichloroaniline and 0.44 µg/mL of p-chloroaniline in 0.2 N hydrochloric acid from Standard stock solution 3
Standard solutions: Prepare Standard solutions 1-9 as listed in Table 2. Transfer the aliquot of the Standard stock solution to each individual 25-mL volumetric flask. Place all flasks in an ice bath at 0° to 5 deg for 10 min. Add to each volumetric flask 1 mL of Sodium nitrite solution, shake well, and let the solution stand for 10 min in the ice bath. Add to each flask 1 mL of the Ammonium sulfamate solution, shake well, and allow the reaction to take place for 10 min inside the ice bath. Add 2 mL of the N-(1-naphthyl)ethylenediamine dihydrochloride solution to each Standard solution 1-8, and remove all volumetric flasks from the ice bath. Do not add the N-(1-naphthyl)ethylenediamine dihydrochloride solution to Standard solution 9. Dilute all solutions with 0.2 N hydrochloric acid to volume. Allow to stand for at least 30 s, and measure the absorbance within 15 min.
Table 2
| Standard Solution | Standard Stock Solution Used | Volume of Standard Stock Solution (mL) | Concentration of Total Chloroaniline (ppm) |
| 1 | Standard stock solution 4 | 2 | 20 |
| 2 | Standard stock solution 4 | 5 | 50 |
| 3 | Standard stock solution 4 | 10 | 100 |
| 4 | Standard stock solution 4 | 15 | 150 |
| 5 | Standard stock solution 3 | 2 | 200 |
| 6 | Standard stock solution 3 | 3 | 300 |
| 7 | Standard stock solution 3 | 4 | 400 |
| 8 | Standard stock solution 3 | 5 | 500 |
| 9 | Standard stock solution 3 | 5 | 0 |
Sample stock solution: Dissolve 500 mg of Triclocarban with 5 mL of the Dimethylformamide solution in a 50-ml beaker, and shake the solution. Continue shaking the solution while adding 15 mL of 0.2 N hydrochloric acid to precipitate the product, and shake for 15 min. Filter the suspension into a 50-mL volumetric flask, and dilute with 0.2 N hydrochloric acid to volume.
Sample solution: Transfer 10 mL of the Sample stock solution to a 25-mL volumetric flask, and place in an ice bath at 0 deg to 5 deg | for 10 min. Add 1 mL of the Sodium nitrite solution, shake well, and let the solution stand for 10 min in the ice bath. Add 1 mL of the Ammonium sulfamate solution, shake well, and allow to stand for 10 min. Add 1 mL of the N-(1-naphthyl) ethylenediamine dihydrochloride solution, and remove the solution from the ice bath. Dilute with 0.2 N hydrochloric acid to volume. Allow to stand for at least 30 s, and measure the absorbance within 15 min.
Sample solution blank: Follow the procedure described in the preparation of the Sample solution but do not add the N-(1-naphthyl)ethylenediamine dihydrochloride solution.
Instrumental conditions
Mode: UV
Analytical wavelength: 552 nm
Analysis
Samples: Standard solutions and Sample solution
Calibrate the absorbance to zero with Standard solution 9. Generate a calibration curve of absorbance against concentration of total chloroaniline using the data from Standard solutions 1-8. Determine the concentration, Cs , in µg/mL of chloroanilines in the Sample solution using the calibration curve. Calculate the concentration, in ppm, of chloroanilines in the portion of Triclocarban taken:
Result = (Cs /Cu ) × F
Cs = concentration of chloroanilines in the Sample solution (µg/mL)
Cu = concentration of Triclocarban in the Sample solution (µg/mL)
F = conversion factor to ppm (106)
Acceptance criteria: NMT 400 ppm
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Sample: Dry a sample at 110° for 4 h.
Acceptance criteria: NMT 0.2%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed container. Store at ambient temperature and humidity.
USP Reference Standards 〈11〉
USP Triclocarban RS
USP Triclocarban Related Compound A RS
1,3-Bis(4-chlorophenyl)urea.
C13H10Cl2N2O 281.14
USP Triclocarban Related Compound C RS
1,3-Bis(3,4-dichlorophenyl)urea.
C13H8Cl4N2O 350.03
USP Triclocarban Related Compound D Mixture RS
Mixture of triarylbiuret 1 (3,5-bis(4-chlorophenyl)-1-(3,4-dichlorophenyl)biuret and triarylbiuret 2 (1,5-bis(4-chlorophenyl)-3-(3,4-dichlorophenyl)biuret).
C20H13Cl4N3O2 469.15


