Triamcinolone Hexacetonide
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
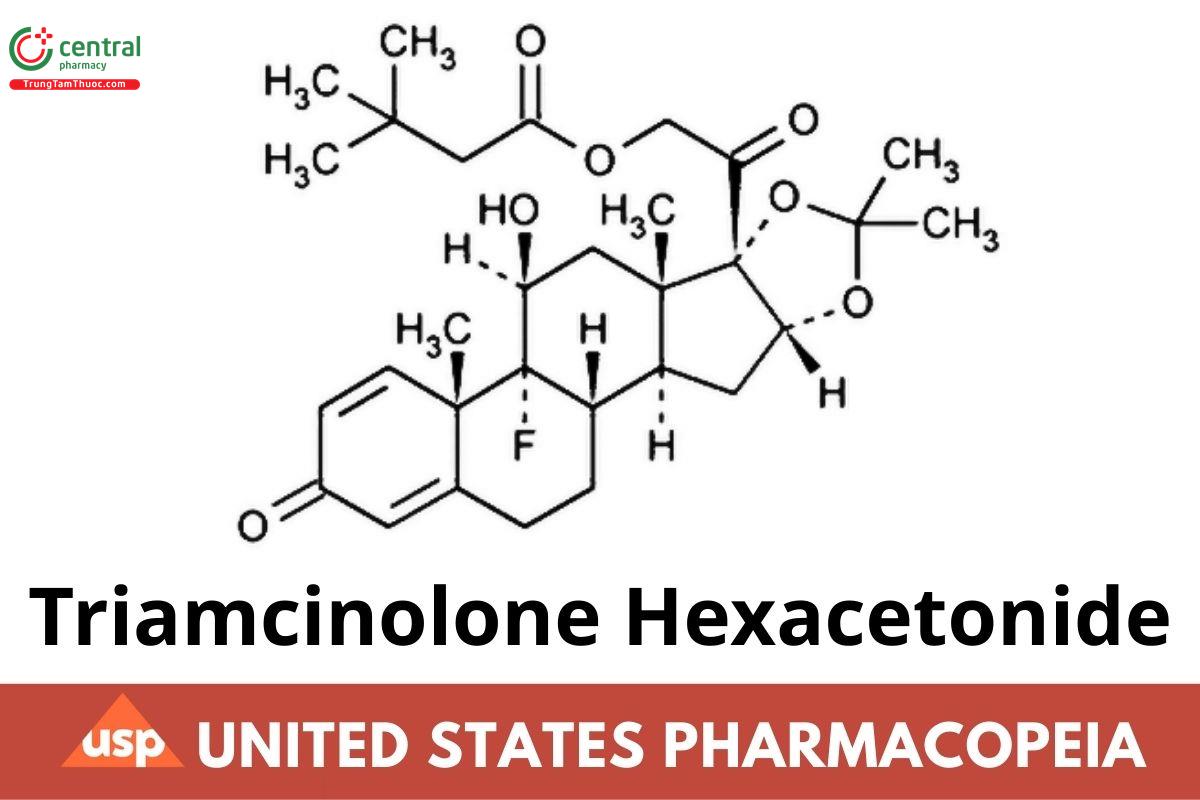
C30H41FO7 532.64
Pregna-1,4-diene-3,20-dione, 21-(3,3-dimethyl-1-oxobutoxy)-9-uoro-11-hydroxy-16,17-[(1-methylethylidene)bis(oxy)]-, (11β,16α)-;
9-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone 21-(3,3-dimethylbutyrate) CAS RN: 5611-51-8; UNII: I7GT1U99Y9.
1 DEFINITION
Triamcinolone Hexacetonide contains NLT 97.0% and NMT 102.0% of C30H41FO7, calculated on the dried basis.
2 IDENTIFICATION
Change to read:
SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020)
3 ASSAY
PROCEDURE
Mobile phase: Methanol and water (3:1)
System suitability solution: 0.4 mg/mL each of USP Triamcinolone Acetonide RS and USP Triamcinolone Hexacetonide RS in methanol
Standard solution: 0.4 mg/mL of USP Triamcinolone Hexacetonide RS in methanol
Sample solution: 0.4 mg/mL of Triamcinolone Hexacetonide in methanol
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm x 25-cm; packing L1
Flow rate: 2 mL/min
Injection size: 10 µL
System suitability
Sample: System suitability solution
[NOTE-The relative retention times for triamcinolone acetonide and triamcinolone hexacetonide are 0.27 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 7.5 between triamcinolone acetonide and triamcinolone hexacetonide
Tailing factor: NMT 1.3 for triamcinolone hexacetonide
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of C30H41FO7 in the portion of Triamcinolone Hexacetonide taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Triamcinolone Hexacetonide RS in the Standard solution (mg/mL)
Cu = concentration of Triamcinolone Hexacetonide in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–102.0% on the dried basis
4 IMPURITIES
Organic Impurities
Procedure: Limit of Triamcinolone Acetonide
Mobile phase, System suitability solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Standard solution: 0.004 mg/mL of USP Triamcinolone Acetonide RS in methanol
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of triamcinolone acetonide in the portion of Triamcinolone Hexacetonide taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of triamcinolone acetonide from the Sample solution
rs = peak response of triamcinolone acetonide from the Standard solution
Cs = concentration of USP Triamcinolone Acetonide RS in the Standard solution (mg/mL)
Cu = concentration of Triamcinolone Hexacetonide in the Sample solution (mg/mL)
Acceptance criteria: NMT 1.0%
5 SPECIFIC TESTS
Optical Rotation, Specific Rotation〈781S〉
Sample solution: 10 mg/mL in chloroform
Acceptance criteria: +91° to +98°
Loss on Drying 〈731〉: Dry a sample in a vacuum at 60° for 4 h: it loses NMT 2.0% of its weight.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at room temperature.
USP Reference Standards 〈11〉
USP Triamcinolone Acetonide RS
USP Triamcinolone Hexacetonide RS


