Triamcinolone Acetonide
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
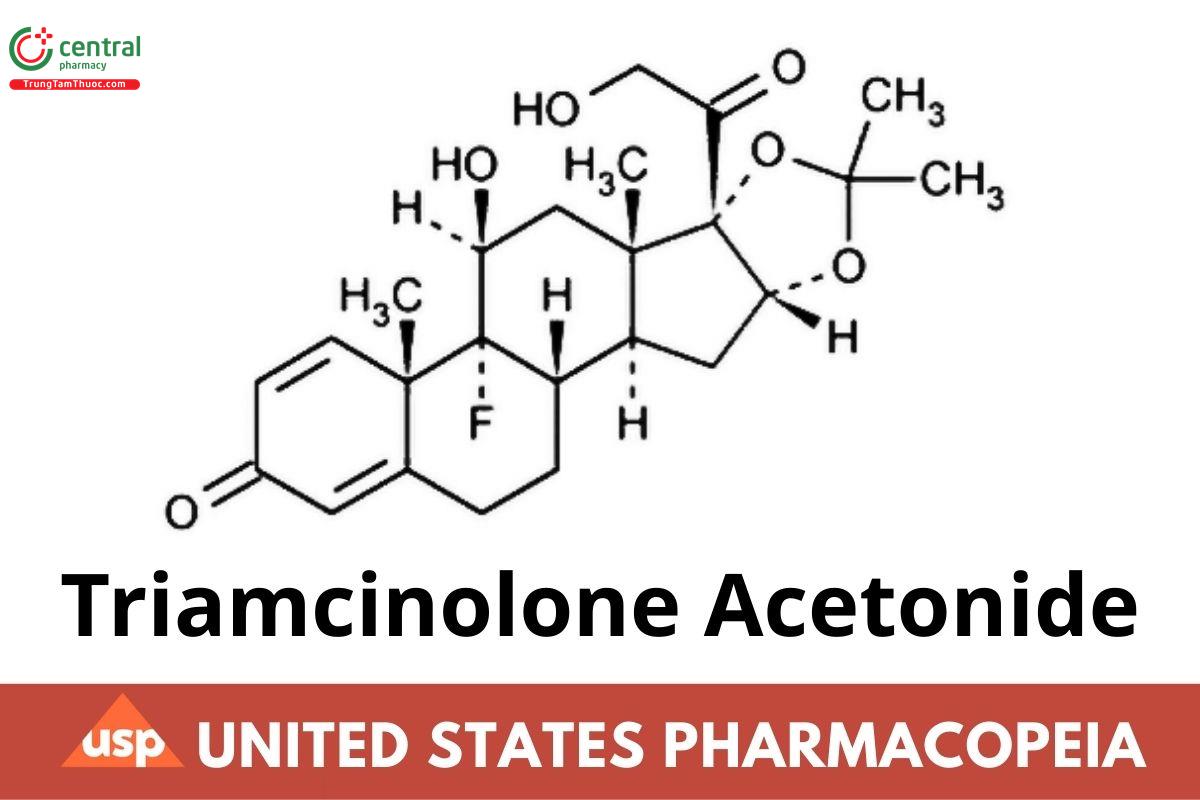
C24H31FO6 434.50
Pregna-1,4-diene-3,20-dione, 9-uoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-, (11β,16α)-.
9-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone CAS RN: 76-25-5; UNII: F446C597KA.
Triamcinolone Acetonide contains not less than 97.0 percent and not more than 102.0 percent of C24H31FO6, calculated on the dried basis.
Packaging and storage—Preserve in well-closed containers. Store at 25°, excursions permitted between 15° and 30°.
USP REFERENCE STANDARDS (11)
USP Fluoxymesterone RS
USP Triamcinolone Acetonide RS
1 Identification
Change to read:
A: Spectroscopic Identification Tests (197). Infrared Spectroscopy. 197K (CN 1-May-2020): recrystallized from methanol.
Change to read:
B: Spectroscopic Identification Tests (197), Ultraviolet-Visible Spectroscopy: 197U (CN 1-May-2020)
Solution: 20 µg per mL.
Medium: methanol.
SPECIFIC ROTATION (7815): between +118° and +130°.
Test solution: 5 mg per mL, in dimethylformamide.
LOSS ON DRYING (731)-Dry it in vacuum at 60° for 4 hours: it loses not more than 1.5% of its weight.
2 Chromatographic purity
Mobile phase-Prepare a filtered and degassed mixture of water and acetonitrile (17:8). Make adjustments if necessary (see System Suitability under Chromatography (621)).
Test solution-Transfer about 25 mg of Triamcinolone Acetonide, accurately weighed, to a 50-mL volumetric flask; dissolve in 25 mL of methanol, shake vigorously to aid dissolution; dilute with Mobile phase to volume; and mix.
Chromatographic system (see CHROMATOGRAPHY (621))-The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm x 30-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. Chromatograph the Test solution, and record the peak responses as directed for Procedure: the resolution, R, between triamcinolone acetonide and any impurity peak is not less than 1.0.
Procedure-Inject about 20 µl of the Test solution into the chromatograph, record the chromatogram for not less than four times the retention time of triamcinolone acetonide, and measure all of the peak responses. Calculate the percentage of each impurity in the portion of Triamcinolone Acetonide taken by the formula:
100(ri/rs)
in which ri is the peak response for each impurity; and rs is the sum of the responses of all the peaks: not more than 0.3% of any individual impurity is found, and not more than 0.8% of total impurities is found.
3 Assay
Mobile phase-Prepare a solution of acetonitrile in water containing approximately 30% (v/v) of acetonitrile.
Internal standard solution-Dissolve USP Fluoxymesterone RS in methanol to obtain a solution having a concentration of about 50 µg per mL.
Standard preparation-Dissolve an accurately weighed quantity of USP Triamcinolone Acetonide RS in Internal standard solution to obtain a solution having a known concentration of about 75 µg per mL. Mix an accurately measured volume of the resulting solution with an equal volume of Mobile phase to obtain a Standard preparation containing about 37.5 µg of USP Triamcinolone Acetonide RS per mL. Assay preparation-Using about 37 mg of Triamcinolone Acetonide, accurately weighed, proceed as directed for Standard preparation. Procedure-Introduce equal volumes (between 15 µL and 25 µL) of the Assay preparation and the Standard preparation into a high-pressure liquid chromatograph (see Chromatography (621)) operated at room temperature, by means of a suitable microsyringe or sampling valve. Adjust the operating parameters with Mobile phase on the column so that the separation of triamcinolone acetonide and internal standard is optimized, with a retention time of about 14.5 minutes for triamcinolone acetonide. Typically, the apparatus is fitted with a 4-mm x 30-cm column containing packing L1 and is equipped with a UV detector capable of monitoring absorbance at 254 nm, and a suitable recorder. In a suitable chromatogram, the coefficient of variation for five replicate injections of a single specimen is not more than 3.0%; and the resolution factor, R (see Chromatography (621)), between the peaks for triamcinolone acetonide and fluoxymesterone is not less than 2.0. Measure the heights of the internal standard and triamcinolone acetonide peaks at the same retention times obtained from the Assay preparation and the Standard preparation. Calculate the quantity, in mg, of C24H31FO6 in the portion of Triamcinolone Acetonide taken by the formula:
1000C(Ru/Rs)
in which C is the concentration, in mg per mL, of USP Triamcinolone Acetonide RS in the Standard preparation; and Ru and Rs are the ratios of the peak heights of triamcinolone acetonide to the internal standard obtained from the Assay preparation and the Standard preparation, respectively.


