Triacetin
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C9H14O6 218.21
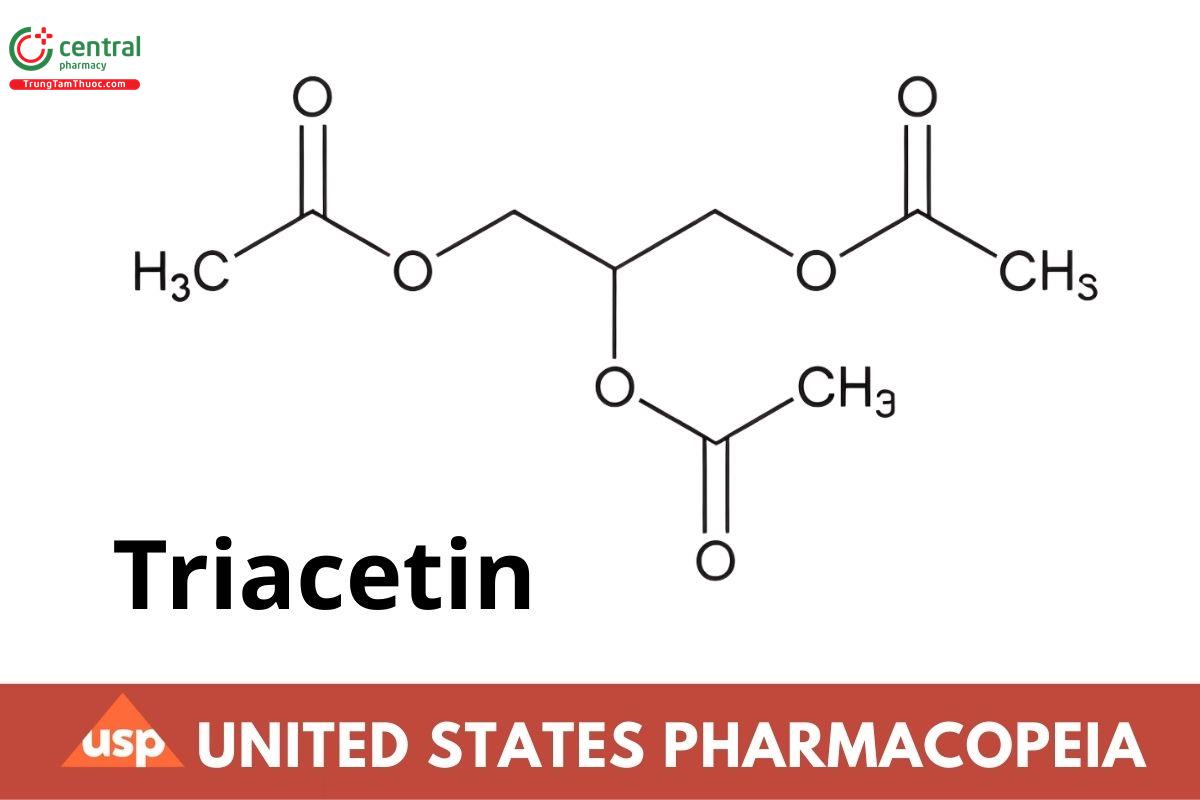
1,2,3-Propanetriol triacetate;
Triacetin;
Glyceryl triacetate CAS RN®: 102-76-1.
1 DEFINITION
Triacetin contains NLT 97.0% and NMT 102.0% of triacetin (C,H,O), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197F▲(CN 1-MAY-2020)
B. The retention time of the triacetin peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Internal standard solution: 0.1 mg/mL of benzyl alcohol in 2-propanol
Standard solution: 0.25 mg/mL of USP Triacetin RS in Internal standard solution
Sample solution: 0.25 mg/mL of Triacetin in Internal standard solution
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.25 - mm x 30 - m coated with a 0.25-µm film of phase G46
Temperatures
Injection port: 200°
Detector: 250°
Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 100 | — | 100 | 1 |
| 100 | 30 | 230 | 1 |
Carrier gas: Hydrogen
Flow rate: 1.5 mL/min
Injection volume: 1 µL
Injection type: Split ratio, 40:1
System suitability
Sample: Standard solution
[NOTE-The relative retention times for benzyl alcohol and triacetin are 0.7 and 1.0, respectively.]
Suitability requirements
Tailing factor: NMT 2.0 for the triacetin peak
Relative standard deviation: NMT 1.0%, peak response ratio of triacetin to benzyl alcohol
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of triacetin (C9H14O6) in the portion of Triacetin taken:
Result = (RU/RS) × (CS/CU) × 100
RU = peak response ratio of triacetin to benzyl alcohol from the Sample solution
RS = peak response ratio of triacetin to benzyl alcohol from the Standard solution
CS = concentration of USP Triacetin RS in the Standard solution (mg/mL)
CU = concentration of Triacetin in the Sample solution (mg/mL)
Acceptance criteria: 97.0%-102.0% on the anhydrous basis
4 IMPURITIES
ORGANIC IMPURITIES
Internal standard solution: 0.02 mg/mL of benzyl alcohol in 2-propanol
Peak identification solution: 0.25 mg/mL each of glycerol, monoacetin, diacetin, and USP Triacetin RS in Internal standard solution
Standard solution: 0.02 mg/mL of USP Triacetin RS in Internal standard solution
Sample solution: 20 mg/mL of Triacetin in Internal standard solution
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.25 - mm x 30 - m coated with a 0.25-µm film of phase G46
Temperatures
Injection port: 200°
Detector: 250°
Column: See Table 2.
Table 2
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 50 | — | 50 | 4 |
| 50 | 30 | 140 | 13 |
| 140 | 30 | 230 | 2 |
Carrier gas: Hydrogen
Flow rate: 1.5 mL/min
Injection volume: 1 µL
Injection type: Split ratio, 40:1
System suitability
Sample: Standard solution
[NOTE-The relative retention times for benzyl alcohol and triacetin are 0.62 and 1.0, respectively.]
Suitability requirements
Tailing factor: NMT 2.0 for the triacetin peak
Relative standard deviation: NMT 5.0%, peak response ratio of triacetin to benzyl alcohol.
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Triacetin taken:
Result = (RU/RS) × (CS/CU) × (1/F) x 100
RU = peak response ratio of each impurity to benzyl alcohol from the Sample solution
RS = peak response ratio of triacetin to benzyl alcohol from the Standard solution
CS = concentration of USP Triacetin RS in the Standard solution (mg/mL)
CU = concentration of Triacetin in the Sample solution (mg/mL)
F = relative response factor (see Table 3)
Acceptance criteria: See Table 3. Reporting threshold: 0.05%.
Table 3
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Acetic acid | 0.24 | 0.62 | 0.1 |
| Glycerol | 0.65 | 0.54 | 0.1 |
| 1-Monoacetin | 0.75 | 1.0 | 0.1 |
| 2-Monoacetin | 0.82 | 1.0 | 0.1 |
| 1,3-Diacetin | 0.90 | 1.0 | 0.1 |
| 1,2-Diacetin | 1.0 | 0.73 | 0.1 |
| Triacetin | 1.0 | — | — |
| Individual, unspecified impurity | — | 1.0 | 0.1 |
| Total impurities | — | — | 0.2 |
5 SPECIFIC TESTS
WATER DETERMINATION (921), Method I: NMT 0.2%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.
USP REFERENCE STANDARDS (11)
USP Triacetin RS


