Tretinoin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
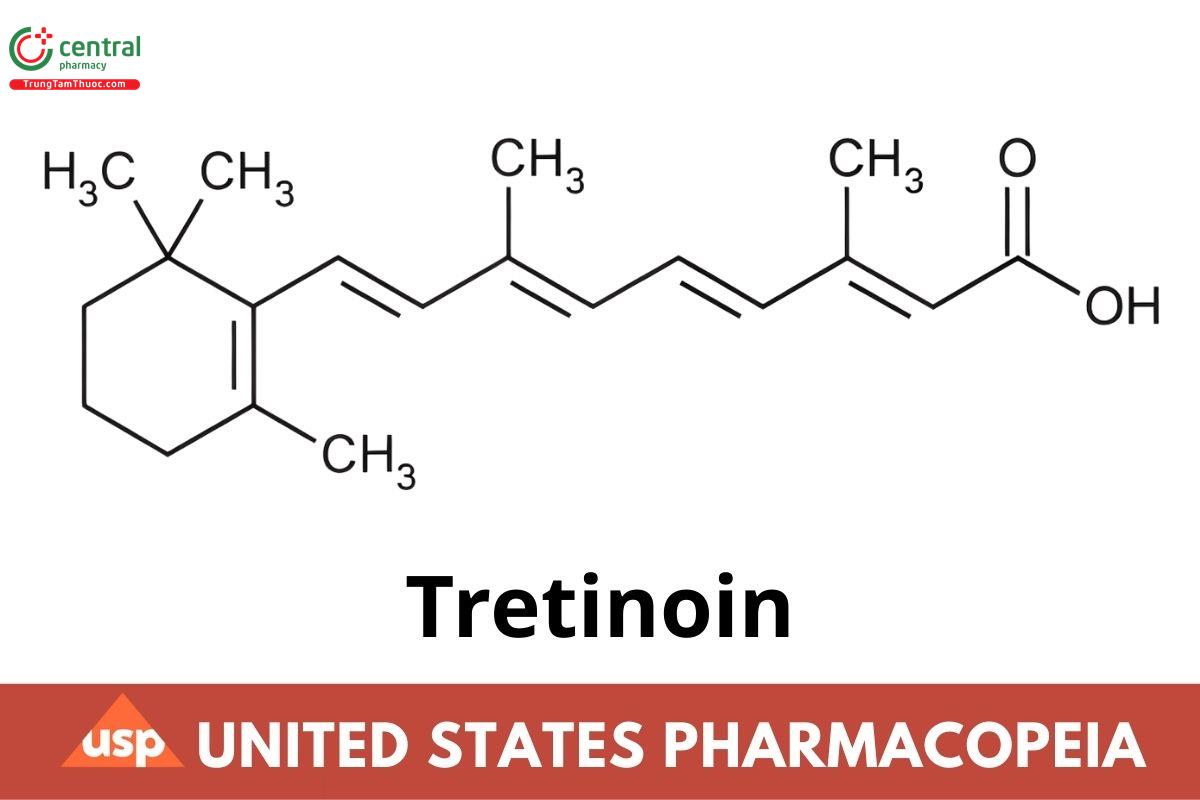
C20H28O2 300.44
Retinoic acid;
all-trans-Retinoic acid CAS RN®: 302-79-4; UNII: 5688UTC01R.
1 DEFINITION
Tretinoin contains NLT 97.0% and NMT 103.0% of tretinoin (C20H28O2), calculated on the dried basis.
Avoid exposure to strong light, and use low-actinic glassware in the performance of the following procedures.
2 IDENTIFICATION
Change to read:
A. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197M▲(CN 1-May-2020)
Change to read:
B. ▲SPECTROSCOPIC IDENTIFICATION TESTS (197), Ultraviolet-Visible Spectroscopy: 1970▲(CN 1-May-2020)
Analytical wavelength: 352 nm
Medium: Dilute 1 mL of 0.01 N hydrochloric acid with isopropyl alcohol to 1000 mL
Sample solution: 4 µg/mL in Medium
Acceptance criteria: Absorptivities do not differ by more than 3.0%, calculated on the dried basis.
3 ASSAY
PROCEDURE
Sample: 240 mg of Tretinoin
Titrimetric system
Mode: Direct titration
Titrant: 0.1 N sodium methoxide VS
Endpoint detection: Visual
Analysis: Dissolve the Sample in 50 mL of dimethylformamide, and add 3 drops of a 1-in-100 solution of thymol blue in dimethylformamide. Titrate with Titrant to a greenish endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N sodium methoxide is equivalent to 30.04 mg of tretinoin (C20H28O2).
Acceptance criteria: 97.0%-103.0% on the dried basis
4 IMPURITIES
4.1 RESIDUE ON IGNITION (281)
NMT 0.1%
4.2 LIMIT OF ISOTRETINOIN
Mobile phase: Isooctane, isopropyl alcohol, and glacial acetic acid (99.65:0.25:0.1)
System suitability stock solution: 250 µg/mL of USP Tretinoin RS in isooctane prepared as follows. Dissolve a quantity of USP Tretinoin RS in a minimum amount of methylene chloride, and add a suitable amount of isooctane to the known concentration.
Standard stock solution: 250 µg/mL of USP Isotretinoin RS in isooctane prepared as follows. Dissolve a quantity of USP Isotretinoin RS in a minimum amount of methylene chloride, and add a suitable amount of isooctane to the known concentration.
System suitability solution: Transfer 5 mL of Standard stock solution into a 100-mL volumetric flask, and add System suitability stock solution to volume.
Standard solution: 12.5 µg/mL of USP Isotretinoin RS in isooctane from Standard stock solution
Sample solution: Transfer 25 mg of Tretinoin into a 100-ml volumetric flask. Dissolve in a minimum amount of methylene chloride, and add isooctane to volume.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 352 nm
Column: 4.0-mm x 25-cm; packing L3
Flow rate: 1 mL/min
Injection volume: 20 µL
System suitability
Sample: System suitability solution
[NOTE-The relative retention times for isotretinoin and tretinoin are about 0.84 and 1.00, respectively.]
Suitability requirements
Resolution: NLT 2.0 between isotretinoin and tretinoin
Relative standard deviation: NMT 2.0% for the isotretinoin peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of isotretinoin in the portion of Tretinoin taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of isotretinoin from the Sample solution
rS = peak response of isotretinoin from the Standard solution
CS = concentration of USP Isotretinoin RS in the Standard solution (µg/mL)
CU = concentration of Tretinoin in the Sample solution (µg/mL)
Acceptance criteria: NMT 5.0%
5 SPECIFIC TESTS
LOSS ON DRYING (731)
Analysis: Dry a sample under vacuum at room temperature for 16 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers, preferably under an atmosphere of an inert gas, protected from light.
USP REFERENCE STANDARDS (11)
USP Isotretinoin RS
USP Tretinoin RS


