Trehalose
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
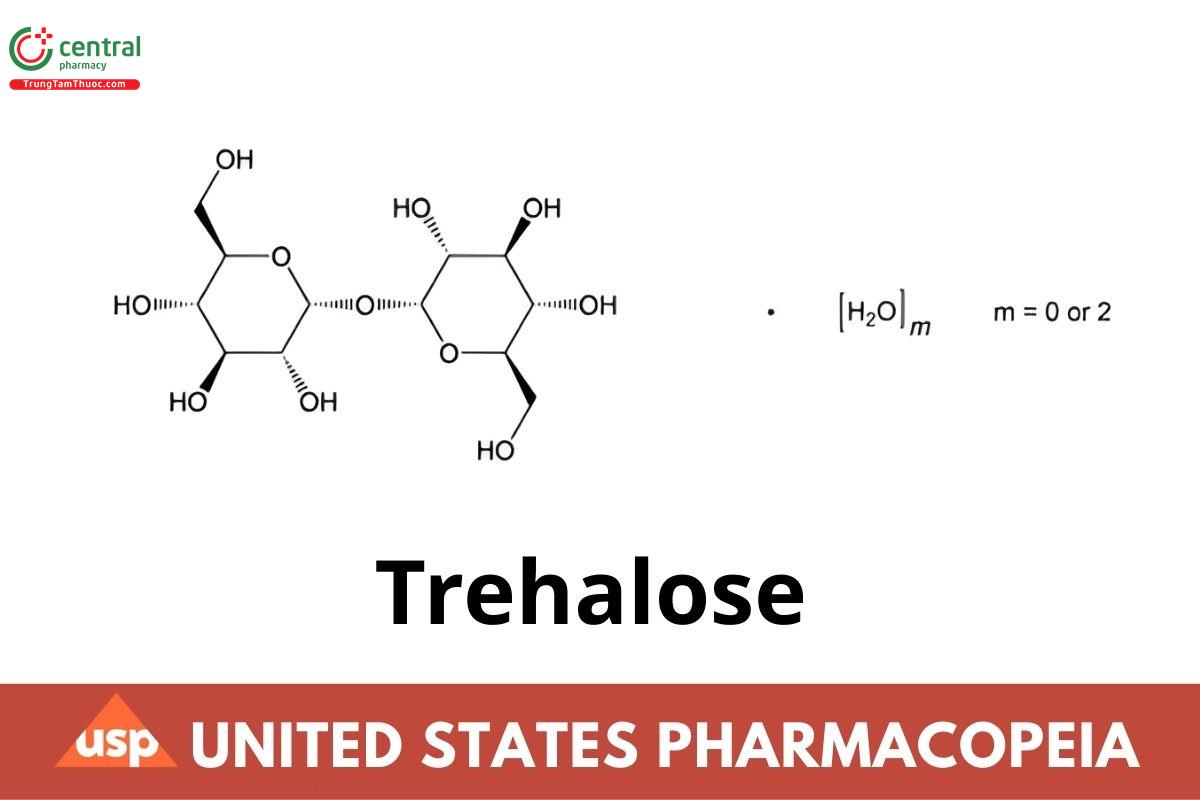
C12H22O11 342.30
C12H22O11 · 2H2O 378.33
α-d-Glucopyranosyl α-d-glucopyranoside anhydrous CAS RN®: 99-20-7.
α-d-Glucopyranosyl α-d-glucopyranoside dihydrate CAS RN®: 6138-23-4.
1 DEFINITION
Trehalose is a stable, nonreducing disaccharide with two Glucose molecules linked in an α,α-1,1 configuration. It is obtained through enzymatic conversion of food-grade starch. It contains NLT 97.0% and NMT 102.0% of trehalose (C12H22O11), calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B.
Sample solution: 400 mg/mL of Trehalose
Analysis: Add 0.4 mL of a solution containing 1-naphthol in 95% alcohol (1 in 20) to 1 mL of the Sample solution. Gently add 2 mL of sulfuric acid to the solution.
Acceptance criteria: A violet color develops at the interface between the two solutions.
C.
Glycine solution: 40 mg/mL of glycine
Sample solution: 40 mg/mL of Trehalose
Analysis: Add 1 mL of diluted hydrochloric acid to 2 mL of the Sample solution. Allow to stand for 20 min at room temperature. Add 4 mL of sodium hydroxide TS and 2 mL of Glycine solution to the Sample solution. Heat the solution for 10 min in boiling water.
Acceptance criteria: A brown color does not develop.
3 ASSAY
Change to read:
Procedure
Mobile phase: Water
Standard solution: Dissolve an accurately weighed quantity of USP Trehalose RS in water to obtain a solution having a concentration of about 10 mg/mL of trehalose. (RB 1-Nov-2024)
Sample solution: 10 mg/mL of Trehalose, calculated on the anhydrous basis
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Refractive index
Column: 8-mm × 30-cm; packing L58
Temperatures
Detector: 40°
Column: 80°
Flow rate: Adjust so that the retention time of trehalose is about 15 min.
Injection volume: 20 μL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of trehalose (C12H22O11) in the portion of Trehalose taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Trehalose RS in the Standard solution (mg/mL)
CU = concentration of Trehalose in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%, determined on 2.0 g of Trehalose
Related Substances
Mobile phase and Chromatographic system: Proceed as directed in the Assay.
Sample solution: 10 mg/mL of Trehalose
System suitability solution: Transfer 2.5 mL of the Sample solution, 25 mg of maltotriose, and 25 mg of glucose to a 10-mL volumetric flask, and dilute with water to volume.
Standard solution: 0.1 mg/mL of the Sample solution
System suitability
Sample: System suitability solution
[Note - The relative retention times for maltotriose, trehalose, and glucose are about 0.9, 1.0, and 1.2, respectively.]
Suitability requirements
Resolution: NLT 1.5 between trehalose and maltotriose
Relative standard deviation: NMT 2.0% for the trehalose peak
Analysis
Samples: Sample solution and Standard solution
Determine the peak areas for all peaks.
Acceptance criteria: For the Sample solution, the areas of any peaks corresponding to maltotriose and other polysaccharrides and eluting before trehalose are NMT half of the area of the peak corresponding to trehalose in the chromatogram of the Standard solution (0.5%). The areas of any peaks corresponding to glucose and eluting after trehalose are NMT half of the area of the peak corresponding to trehalose inthe chromatogram of the Standard solution (0.5%).
5 SPECIFIC TESTS
Color and Clarity of Solution
Sample solution: 33 g of Trehalose in 67 g of recently boiled water
Analysis: Using a suitable spectrophotometer (see Ultraviolet-Visible Spectroscopy 〈857〉), measure the absorbances of the Sample solution at 420 and 720 nm in a 10-cm cuvette. The absorbance of the Sample solution at 720 nm is NMT 0.050.
Determine the absorbance difference:
Result = A420 − A720
A420 = absorbance of the Sample solution at 420 nm
A720 = absorbance of the Sample solution at 720 nm
Acceptance criteria: The absorbance difference is NMT 0.100.
Optical Rotation, Specific Rotation 〈781S〉
Sample solution: 100 mg/mL
Acceptance criteria: +197° to +201° at 20°
Microbial Enumeration Tests 〈61〉 and Tests for Specified Microorganisms 〈62〉: The total aerobic microbial count is NMT 100 cfu/g, and the total combined molds and yeasts count is NMT 100 cfu/g. It meets the requirements of the tests for absence of Salmonella species and Escherichia coli.
pH 〈791〉
Sample solution: 100 mg/mL
Acceptance criteria: 4.5–6.5
Water Determination, Method I 〈921〉
Sample: 0.1 g
Acceptance criteria
Anhydrous: NMT 1.0%
Dihydrate: 9.0%–11.0%
Bacterial Endotoxins Test 〈85〉: If labeled for use in preparing parenteral dosage forms, it also meets the following requirements. The level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Trehalose is used can be met. Where the label states that Trehalose must be subjected to further processing during the preparation of injectable dosage forms, the level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s) in which Trehalose is used can be met.
Chloride and Sulfate, Chloride 〈221〉
Sample: 2.0 g
Acceptance criteria: No more chloride than corresponds to 0.70 mL of 0.01 M hydrochloric acid (NMT 0.0125%)
Chloride and Sulfate, Sulfate 〈221〉
Sample: 2.0 g
Acceptance criteria: No more sulfate than corresponds to 0.83 mL of 0.005 M sulfuric acid (NMT 0.0200%)
Nitrogen Determination, Method II 〈461〉
Sample: 5.0 g
Analysis: Proceed as directed in Method II, except increase the volume of sulfuric acid for digestion to 30 mL and the volume of the sodium hydroxide solution (2 in 5) to 45 mL.
Acceptance criteria: NMT 0.005%
Soluble Starch
Sample solution: 10% Trehalose (w/v)
Analysis: Add several drops of iodine TS to the Sample solution.
Acceptance criteria: No blue color develops.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. No storage requirements specified.
Labeling: Where Trehalose is intended for use in the manufacture of injectable dosage forms, it is so labeled. Where Trehalose must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of bacterial endotoxins, it is so labeled.
USP Reference Standards 〈11〉
USP Trehalose RS


