Tramadol Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
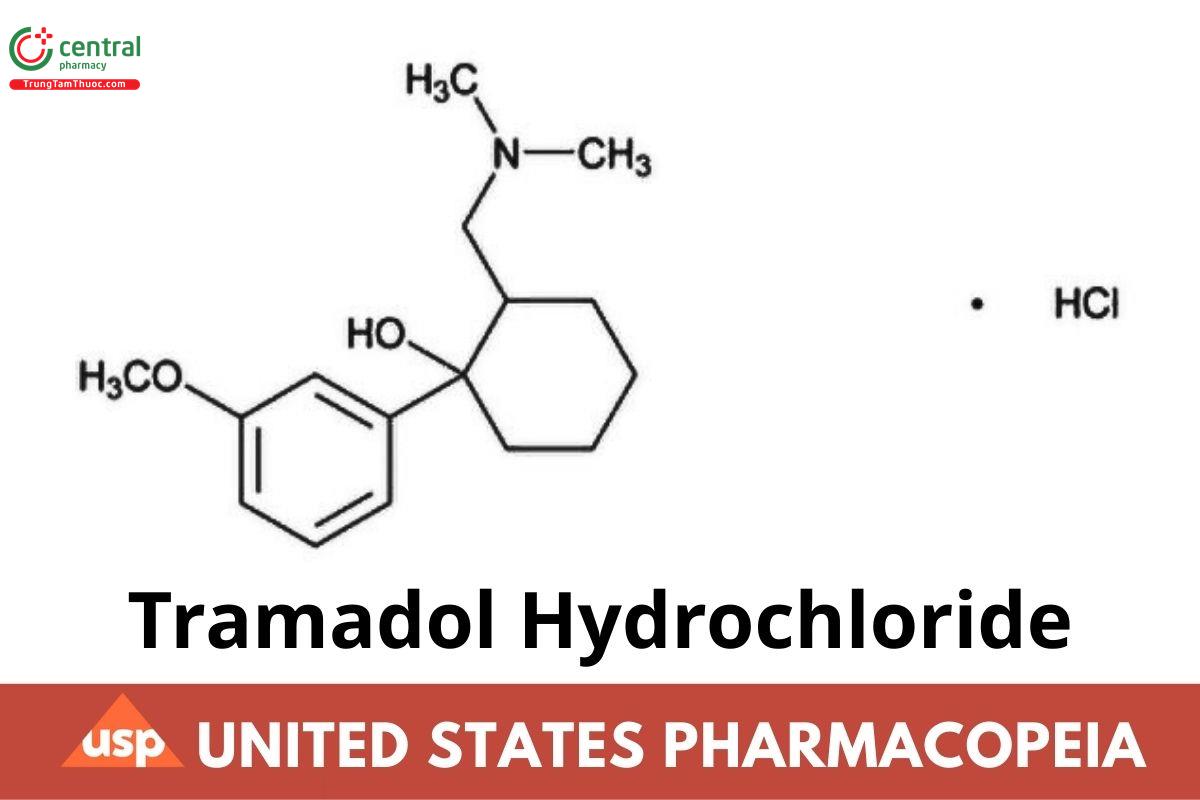
C₁₆H₂₅NO₂ · HCl 299.84
(±)-cis-2-[(Dimethylamino)methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride;
(±)-cis-2-[(Dimethylamino)methyl]-1-(m-methoxyphenyl) cyclohexanol hydrochloride CAS RN®: 36282-47-0; UNII: 9N7R477WCK.
1 DEFINITION
Tramadol Hydrochloride contains NLT 98.0% and NMT 102.0% of tramadol hydrochloride (C₁₆H₂₅NO₂ · HCl), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. Identification Tests-General, Chloride 〈191〉: An aqueous solution (1 in 100) meets the requirements.
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Solution A: Dissolve 2 mL of trifluoroacetic acid in 1000 mL of water.
Mobile phase: Acetonitrile and Solution A (30:70)
System suitability solution: 0.05 mg/mL each of USP Tramadol Hydrochloride RS and USP Tramadol Related Compound A RS in Mobile phase
Standard solution: 1.5 mg/mL of USP Tramadol Hydrochloride RS in Mobile phase
Sample solution: 1.5 mg/mL of Tramadol Hydrochloride in Mobile phase
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 270 nm
- Column: 4.6-mm × 25-cm; 5-µm packing L1
- Flow rate: 1 mL/min
- Injection volume: 20 µL
System suitability
- Sample: System suitability solution
- [Note-The relative retention times for tramadol related compound A and tramadol are about 0.9 and 1.0, respectively.]
- Suitability requirements
- Resolution: NLT 2.0 between tramadol related compound A and tramadol
- Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of tramadol hydrochloride (C₁₆H₂₅NO₂ · HCl) in the portion of Tramadol Hydrochloride taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × 100
rᵤ = peak response of tramadol from the Sample solution
rₛ = peak response of tramadol from the Standard solution
Cₛ = concentration of USP Tramadol Hydrochloride RS in the Standard solution (mg/mL)
Cᵤ = concentration of Tramadol Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
4.1 Residue on Ignition 〈281〉
NMT 0.1%
4.2 Content of Chloride
Sample solution: 150 mg of Tramadol Hydrochloride in 40 mL of water
Analysis: To the Sample solution add, with stirring, 7.5 mL of 4 N nitric acid and 15.0 mL of 0.1 N silver nitrate, and titrate with 0.1 N ammonium thiocyanate VS, determining the endpoint potentiometrically, and using a silver–glass electrode system. Each mL of 0.1 N ammonium thiocyanate is equivalent to 3.545 mg of chloride.
Acceptance criteria: 11.6%–12.1% of chloride is found.
4.3 Limit of Tramadol Related Compound B (2-[(Dimethylamino)methyl]cyclohexanone hydrochloride)
Standard solution: 0.1 mg/mL of USP Tramadol Related Compound B RS in methanol
Sample solution: 50 mg/mL of Tramadol Hydrochloride in methanol
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Developing solvent system: Toluene, isopropyl alcohol, and 25% ammonia water (80:19:1)
Sodium nitrite solution: 50 mg/mL of sodium nitrite in water
Analysis: Place the plate in a chromatographic chamber saturated with ammonia vapor from stronger ammonia water, and allow to stand for NLT 20 min. Separately apply about 10 µL each of the Sample solution and the Standard solution to the plate, and develop the plate until the solvent front is NLT 10 cm above the line of application. Remove the plate, spray with Dragendorff's TS, and air-dry for 5 min. Spray the dried plate with Sodium nitrite solution until the spot from tramadol related compound B in the Standard solution is visible. Any secondary spot from the Sample solution corresponding to tramadol related compound B is not more intense than a corresponding spot from the Standard solution.
Acceptance criteria: NMT 0.2%
4.4 Organic Impurities
Mobile phase, System suitability solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Tramadol Hydrochloride taken:
Result = (rU/rT) × 100
rU = peak response of each impurity from the Sample solution
rT = sum of all the peak responses from the Sample solution
Acceptance criteria
- Tramadol related compound A: NMT 0.2%
- Individual impurities: NMT 0.1%
- Total impurities: NMT 0.4%
5 SPECIFIC TESTS
5.1 Water Determination, Method Ia 〈921〉
NMT 0.5%
5.2 Acidity
Sample solution: 500 mg of Tramadol Hydrochloride in 10 mL of water
Analysis: To the Sample solution add 0.2 mL of methyl red TS and 0.2 mL of 0.01 N hydrochloric acid VS, and titrate with 0.01 N sodium hydroxide VS.
Acceptance criteria: NMT 0.4 mL of 0.01 N sodium hydroxide VS is required to produce a yellow color.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers, and store at controlled room temperature.
USP Reference Standards 〈11〉
USP Tramadol Hydrochloride RS
USP Tramadol Related Compound A RS
(RS,SR)-1-(3-Methoxyphenyl)-2-(dimethylaminomethyl)cyclohexanol hydrochloride.
C₁₆H₂₅NO₂ · HCl 299.84
USP Tramadol Related Compound B RS
(2-[(Dimethylamino)methyl]cyclohexanone hydrochloride).
C₉H₁₇NO · HCl 191.70


