Tolterodine Tartrate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
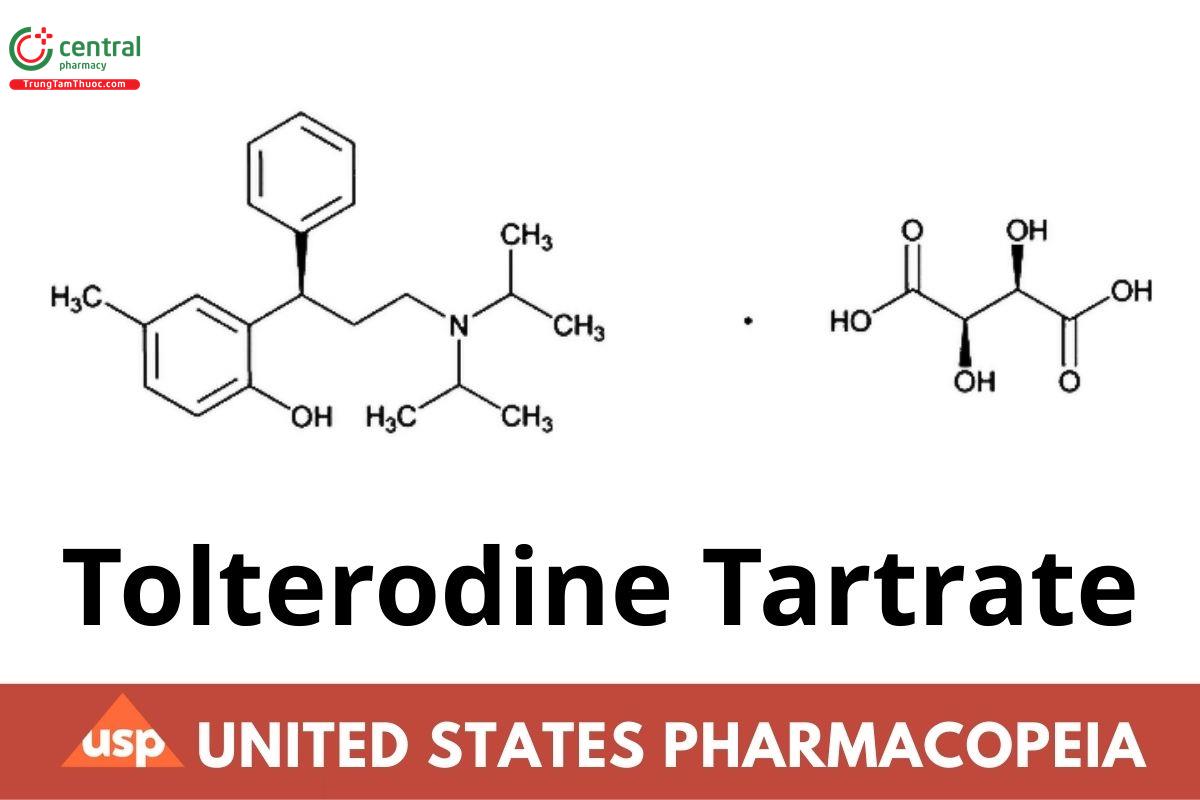
C22H31NO · C4H6O6 475.57
(R)-2-[3-[Bis(1-methylethyl)amino]-1-phenylpropyl]-4-methylphenol (2R,3R)-2,3-dihydroxybutanedioate (1:1) (salt);
(+)-(R)-2-[α-[2-(Diisopropylamino)ethyl]benzyl]-p-cresol l-tartrate (1:1) (salt);
(R)-2-[3-(Diisopropylamino)-1-phenylpropyl]-4-methylphenol tartrate CAS RN: 124937-52-6; UNII: 5T619TQR3R.
1 DEFINITION
Tolterodine Tartrate contains NLT 97.0% and NMT 103.0% of tolterodine tartrate (C22H31NO · C4H6O6), calculated on the as-is basis.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197K (CN 1-MAY-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Mobile phase: Acetonitrile, water, and phosphoric acid (330:670:1)
Standard solution: 0.35 mg/mL of USP Tolterodine Tartrate RS in Mobile phase
Sample solution: 0.35 mg/mL of Tolterodine Tartrate in Mobile phase
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 285 nm
Column: 4.6-mm x 6-mm x 25-cm; 5-µm packing 11
Flow rate: 1.0 mL/min
Injection volume: 5 µL
System suitability
Sample: Standard solution
Suitability requirements
Relative standard deviation: NMT 1.0% from six replicate injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of tolterodine tartrate (C22H31NO · C4H6O6) in the portion of Tolterodine Tartrate taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response from the Sample solution
rs = peak response from the Standard solution
Cs = concentration of USP Tolterodine Tartrate RS in the Standard solution (mg/mL)
Cu = concentration of Tolterodine Tartrate in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–103.0% on the as-is basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Organic Impurities
Solution A: Acetonitrile, water, and perchloric acid (100:900:1.5)
Solution B: Acetonitrile, water, and perchloric acid (500:500:1.5)
Solution C: Acetonitrile
Mobile phase: See Table 1. Return to original conditions, and re-equilibrate the system.
Table 1
| Time (min) | Solution A (%) | Solution B (%) | Solution C (%) |
| 0 | 75 | 25 | 0 |
| 5 | 75 | 25 | 0 |
| 22 | 0 | 100 | 0 |
| 47 | 0 | 0 | 100 |
| 57 | 0 | 0 | 100 |
Diluent: Acetonitrile and water (50:50)
System suitability solution: 10 mg/mL of USP Tolterodine System Suitability Mixture RS in Diluent. See Table 2 for relative retention times of the main components of the mixture.
Table 2
| Component of USP Tolterodine System Suitability Mixture RS | Relative Retention Time |
| p-Cresol | 0.75 |
| trans-Cinnamic acid | 0.81 |
| Monoisopropyl tolterodine | 0.88 |
| Tolterodine | 1.0 |
| Diol impurity | 1.18 |
| Tolterodine dimera | 1.44 |
| 6-Methyl-4-phenylchroman-2-ola | 1.48 |
| Diol acetate impurity | 1.54 |
| 6-Methyl-4-phenylchroman-2-one | 1.59 |
a Undened stereochemistry.
Standard solution: 0.01 mg/mL of USP Tolterodine Tartrate RS in Diluent
Sensitivity solution: 0.005 mg/mL of USP Tolterodine Tartrate RS in Diluent from the Standard solution
Sample solution: 10 mg/mL of Tolterodine Tartrate in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 5-µm packing L1
Column temperature: 65°
Flow rate: 1.0 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
Suitability requirements
Resolution: NLT 1.5 between diol acetate impurity and 6-methyl-4-phenylchroman-2-one, System suitability solution
Relative standard deviation: NMT 3.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Tolterodine Tartrate taken:
Result = (ru /rs ) × (Cs /Cu ) × (1/F) × 100
ru = peak response for each impurity from the Sample solution
rs = peak response of tolterodine from the Standard solution
Cs = concentration of USP Tolterodine Tartrate RS in the Standard solution (mg/mL)
Cu = concentration of Tolterodine Tartrate in the Sample solution (mg/mL)
F = relative response factor (see Table 3)
Acceptance criteria: See Table 3. Disregard any peak below 0.05% and any peak eluting at retention times of less than 4 min.
Table 3
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
| Monoisopropyl tolterodine | 0.88 | 1.6 | 0.25 |
| Tolterodine | 1.0 | - | - |
| 6-Methyl-4-phenylchroman-2-ol | 1.48 | 1.9 | 0.25 |
| Any other individual impurity | - | 1.0 | 0.1 |
| Total impurities | - | - | 0.5 |
ENANTIOMERIC PURITY
Buffer: Prepare pH 7.1 buffer as follows. Transfer 21.0 mL of 1 M solution of monobasic sodium phosphate and 53.3 mL of 0.5 M solution of dibasic sodium phosphate dihydrate to a 1000-mL volumetric flask, and dilute with water to volume. Dilute 100.0 mL of this solution with water to 1000.0 mL
Mobile phase: Add 0.97 g of tetrabutylammonium bromide to a mixture of 930 mL of Buffer and 70 mL of isobutyl alcohol.
System suitability solution: 0.02 mg/mL each of USP Tolterodine Tartrate RS and USP Tolterodine S-Enantiomer RS in Mobile phase
Standard solution: 0.0004 mg/mL of USP Tolterodine S-Enantiomer RS in Mobile phase
Sample solution: 0.04 mg/mL of Tolterodine Tartrate in Mobile phase
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 2-mm x 10-cm; 5-um packing L41
Flow rate: 0.2 mL/min
Injection volume: 20 µL
System suitability
Sample: System suitability solution
[NOTE-The relative retention times for tolterodine S-enantiomer and tolterodine are 0.9 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 1.4 between tolterodine S-enantiomer and tolterodine
Column efficiency: NLT 1500 theoretical plates for tolterodine
Relative standard deviation: NMT 3% for each of tolterodine S-enantiomer and tolterodine
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of tolterodine S-enantiomer in the portion of Tolterodine Tartrate taken:
Result = (ru /rs ) × (Cs /Cu ) × 100
ru = peak response of tolterodine S-enantiomer from the Sample solution
rs = peak response of tolterodine S-enantiomer from the Standard solution
Cs = concentration of USP Tolterodine S-Enantiomer RS in the Standard solution (mg/mL)
Cu = concentration of Tolterodine Tartrate in the Sample solution (mg/mL)
Acceptance criteria: NMT 1.0%
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry under vacuum at 100° for 2 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at room temperature.
USP Reference Standards 〈11〉
USP Tolterodine S-Enantiomer RS
(S)-2-[3-(Diisopropylamino)-1-phenylpropyl]-4-methylphenol tartrate.
C22H31NO · C4H6O6 475.57
USP Tolterodine System Suitability Mixture RS
The mixture contains tolterodine tartrate and the following impurities (other impurities may also be present):
p-Cresol.
C7H8O 108.14
trans-Cinnamic acid. C9H8O2 148.16
Monoisopropyl tolterodine;
(R)-2-[3-(Isopropylamino)-1-phenylpropyl]-4-methylphenol. C19H25NO 283.41
Diol impurity;
2-(3-Hydroxy-1-phenylpropyl)-4-methylphenol. C16H18O2 242.32
Tolterodine dimer;
N,N-Bis[3-(2-hydroxy-5-methylphenyl)-3-phenylpropyl]-N-isopropylamine. C35H41NO2 507.72
6-Methyl-4-phenylchroman-2-ol. C16H16O2 240.30
Diol acetate impurity;
3-(2-Hydroxy-5-methylphenyl)-3-phenylpropyl acetate. C18H22O3 284.35
6-Methyl-4-phenylchroman-2-one. C16H14O2 238.29
USP Tolterodine Tartrate RS


