Tobramycin
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
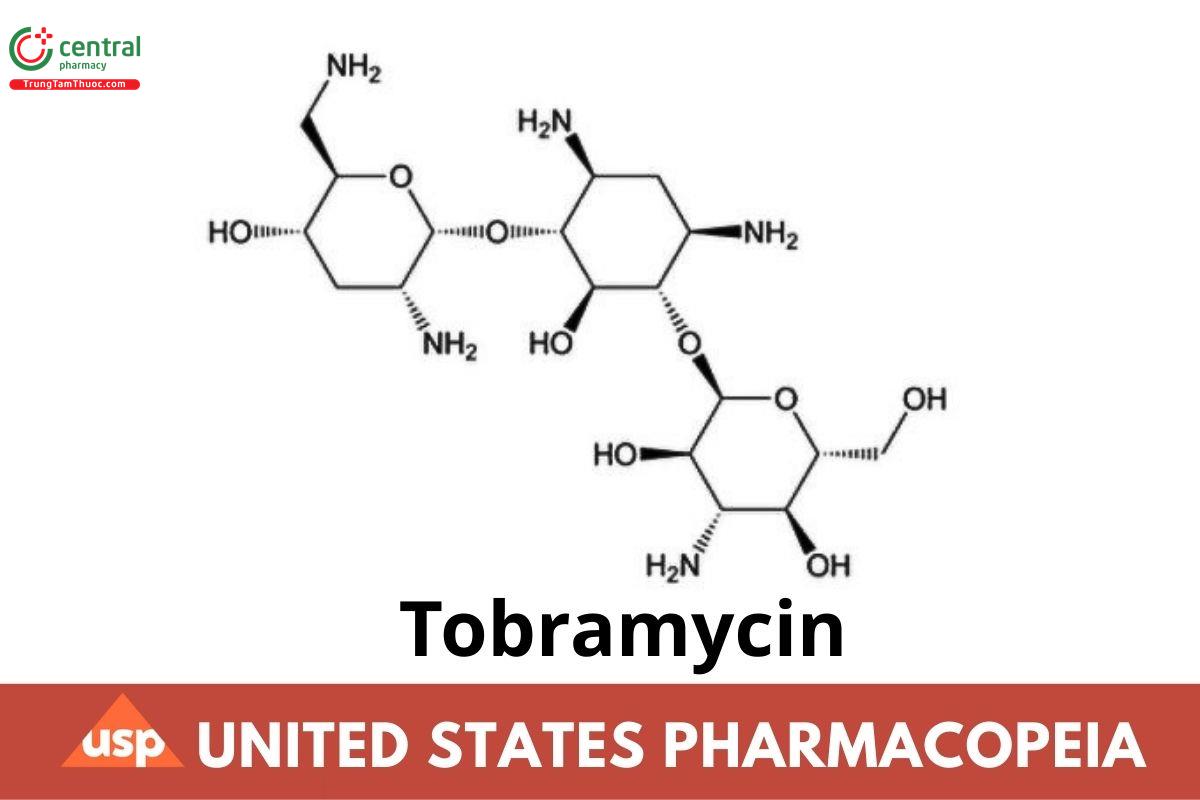
C₁₈H₃₇N₅O₉ 467.51
d-Streptamine, O-3-amino-3-deoxy-α-d-glucopyranosyl-(1→6)-O-[2,6-diamino-2,3,6-trideoxy-α-d-ribo-hexopyranosyl-(1→4)]-2-deoxy-;
O-3-Amino-3-deoxy-α-d-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6-trideoxy-α-d-ribo-hexopyranosyl-(1→6)]-2-deoxy-l-streptamine
CAS RN®: 32986-56-4; UNII: VZ8RRZ51VK.
1 DEFINITION
Tobramycin has a potency of NLT 900 µg/mg of tobramycin (C₁₈H₃₇N₅O₉), calculated on the anhydrous basis.
2 IDENTIFICATION
2.1 A.
Standard solution: 6 mg/mL of USP Tobramycin RS in water
Sample solution: 6 mg/mL of Tobramycin in water
Solution A: Standard solution and Sample solution (1:1)
Chromatographic system
- (See Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography.)
- Mode: TLC
- Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
- Application volume: 3 µL
- Developing solvent system: Methanol, chloroform, and ammonium hydroxide (60:25:30)
- Diluent: Butyl alcohol and pyridine (100:1)
- Spray reagent: 10 mg/mL of ninhydrin in Diluent
Analysis
Samples: Standard solution, Sample solution, and Solution A
Apply the Samples to the plate. Place the plate in a suitable chromatographic chamber, and develop the chromatogram in the Developing solvent system until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, allow the solvent to evaporate, and heat the plate at 110° for 15 min. Immediately locate the spots on the plate by spraying with Spray reagent.
Acceptance criteria: Tobramycin appears as a pink spot, and the Rf values of the spots from the Sample solution and from Solution A, respectively, correspond to those obtained from the Standard solution.
2.2 B.
The retention time of the major peak of the Derivatized sample solution corresponds to that of the Derivatized standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Mobile phase: Dissolve 2.0 g of tris(hydroxymethyl)aminomethane in 800 mL of water. Add 20 mL of 1 N sulfuric acid, and dilute with acetonitrile to obtain 2000 mL of solution. Cool, and pass through a filter of 0.2-µm or finer pore size.
Solution A: 10 mg/mL of 2,4-dinitrofluorobenzene in alcohol. This solution may be used for 5 days if refrigerated when not in use.
Solution B: 15 mg/mL of tris(hydroxymethyl)aminomethane in water. This solution may be used for 1 month if refrigerated when not in use.
Solution C: 3 mg/mL of tris(hydroxymethyl)aminomethane prepared as follows. Transfer 40 mL of Solution B to a 200-mL volumetric flask. Add Dimethyl sulfoxide with mixing, and dilute with dimethyl sulfoxide to volume. Use this reagent within 4 h. If kept immersed in an ice-water bath below 10°, the reagent may be used for up to 8 h.
Standard stock solution: 1.1 mg/mL of USP Tobramycin RS prepared as follows. Transfer 55 mg of USP Tobramycin RS to a 50-mL volumetric flask. Add 1 mL of 1 N sulfuric acid and enough water to dissolve it, and dilute with water to volume.
Standard solution: 0.22 mg/mL of USP Tobramycin RS from Standard stock solution in water
Sample stock solution: 1.1 mg/mL of Tobramycin prepared as follows. Transfer 55 mg of Tobramycin to a 50-mL volumetric flask. Add 1 mL of 1 N sulfuric acid and enough water to dissolve it, and dilute with water to volume.
Sample solution: 0.22 mg/mL of Tobramycin from Sample stock solution in water
Derivatized standard solution, Derivatized sample solution, and Blank solution: Proceed as follows. Heat all solutions at the same temperature and for the same duration of time as indicated. Move all flasks to and from the 60° constant temperature bath at the same time.
To separate 50-mL volumetric flasks transfer 4.0 mL of the Standard solution, 4.0 mL of the Sample solution, and 4.0 mL of water. To each flask add 10 mL of Solution A and 10 mL of Solution C, shake, and insert the stopper. Place the flasks in a constant temperature bath at 60 ± 2°, and heat for 50 ± 5 min. Remove the flasks from the bath, and allow to stand for 10 min. Add acetonitrile to about 2 mL below the 50-mL mark, allow to cool to room temperature, then dilute with acetonitrile to volume. The solutions thus obtained are the Derivatized standard solution, the Derivatized sample solution, and the Blank solution, respectively.
System suitability stock solution: 0.24 mg/mL of p-naphtholbenzein in acetonitrile
System suitability solution: Transfer 2 mL of the System suitability stock solution to a 10-mL volumetric flask, dilute with Derivatized standard solution to volume, and use promptly.
Chromatographic system
- (See Chromatography 〈621〉, System Suitability.)
- Mode: LC
- Detector: UV 365 nm
- Column: 3.9-mm × 30-cm; packing L1
- Flow rate: 1.2 mL/min
- Injection volume: 20 µL
System suitability
- Samples: Derivatized standard solution and System suitability solution
- [Note-The relative retention times for p-naphtholbenzein and tobramycin are about 0.6 and 1.0, respectively.]
- Suitability requirements
- Resolution: NLT 4.0 between p-naphtholbenzein and tobramycin, System suitability solution
- Relative standard deviation: NMT 2.0% for tobramycin, Derivatized standard solution
Analysis
Samples: Derivatized standard solution, Derivatized sample solution, and Blank solution
Use the Blank solution to identify the solvent and reagent peaks.
Calculate the quantity, in µg/mg, of tobramycin (C₁₈H₃₇N₅O₉) in the portion of Tobramycin taken:
Result = (rᵤ/rₛ) × (Cₛ/Cᵤ) × P
rᵤ = peak area of tobramycin from the Derivatized sample solution
rₛ = peak area of tobramycin from the Derivatized standard solution
Cₛ = concentration of USP Tobramycin RS in the Standard solution (mg/mL)
Cᵤ = concentration of Tobramycin in the Sample solution (mg/mL)
P = potency of tobramycin in USP Tobramycin RS (µg/mg)
Acceptance criteria: NLT 900 µg/mg of tobramycin (C₁₈H₃₇N₅O₉) on the anhydrous basis
4 IMPURITIES
4.1 Residue on Ignition 〈281〉
Analysis: Moisten the charred residue with 2 mL of nitric acid and 5 drops of sulfuric acid.
Acceptance criteria: NMT 1.0%
4.2 Organic Impurities
Solution A: Dilute 20 mL of sodium hypochlorite solution with water to 100 mL.
Solution B: Dissolve 1.1 g of potassium iodide in 60 mL of water, boil for 15 min, and slowly add a suspension of 1.5 g of soluble starch in 10 mL of water. Add 25 mL of water, and boil for 10 min. Allow to cool, and dilute with water to 100 mL.
Solution C: 29.2 g of sodium chloride in 100 mL of water
Sample solution: Transfer 50 mg of Tobramycin to a 10-mL volumetric flask. Dissolve in 7 mL of water, and adjust with 1 N sulfuric acid to a pH of 5.5 ± 0.4. Dilute with water to volume.
Standard solution: 0.05 mg/mL of Tobramycin from Sample solution in water
Chromatographic system
- (See Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography.)
- Mode: TLC
- Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
- Application volume: 1 µL
- Developing solvent system: Alcohol, Solution C, and water (30:50:20)
Analysis
Samples: Sample solution and Standard solution
Apply the Samples to the plate. Develop the chromatogram in a saturated chromatographic chamber containing the Developing solvent system until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chromatographic chamber, evaporate the solvent in a current of hot air, then heat the plate at 110° for 10 min. Lightly spray the hot plate with Solution A. Dry the plate in a current of cold air until a sprayed area of the plate below the origin gives at most a faint blue color with a drop of Solution B. Then spray the plate with Solution B.
Acceptance criteria: Bluish-purple spots are immediately visible. Other than the principal tobramycin spot, no spot observed in the Sample solution is more intense than the principal spot from the Standard solution (1.0%).
5 SPECIFIC TESTS
5.1 pH 〈791〉
Sample solution: 100 mg/mL
Acceptance criteria: 9.0–11.0
5.2 Water Determination 〈921〉, Method I
Analysis: Use a mixture of formamide and methanol (1:3) as the solvent.
Acceptance criteria: NMT 8.0%
5.3 Sterility Tests 〈71〉
Where the label states that Tobramycin is sterile, it meets the requirements.
5.4 Bacterial Endotoxins Test 〈85〉
Where the label states that Tobramycin is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 2.00 USP Endotoxin Units/mg of tobramycin. Where it is intended for use in preparing ophthalmic dosage forms, it is exempt from the requirements for Bacterial Endotoxins Test.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: Where it is intended for use in preparing injectable or ophthalmic dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable or ophthalmic dosage forms.
USP Reference Standards 〈11〉
USP Tobramycin RS


