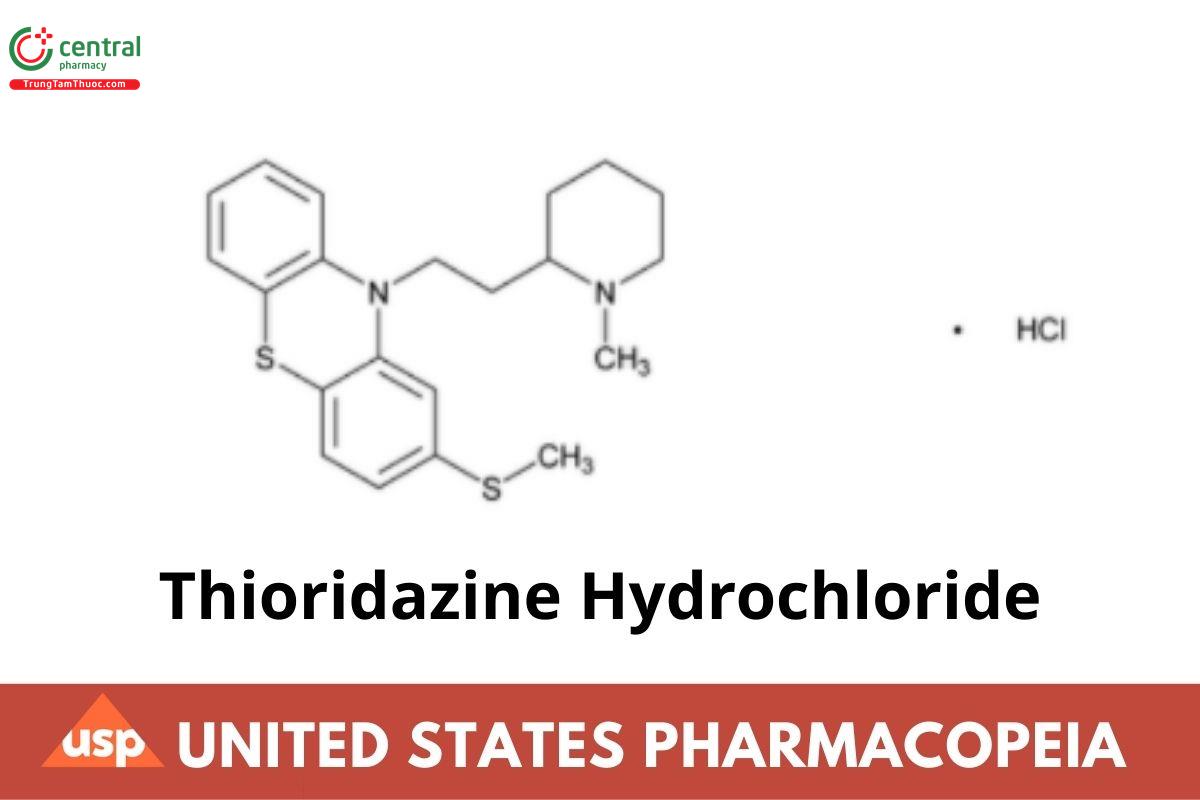
Thioridazine Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
Thioridazine Hydrochloride contains NLT 99.0% and NMT 101.0% of thioridazine hydrochloride, calculated on the dried basis.
[Note—Throughout the following procedures, protect test or Assay samples, the Reference Standard, and solutions containing them, by conducting the analyses without delay, under subdued light, or by using low-actinic glassware.]
1 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. Identification Tests—General 〈191〉, Chemical Identification Tests, Chloride
Sample solution: 10 mg/mL in a mixture of water and alcohol (50:50)
Acceptance criteria: Meets the requirements of the test for amine hydrochlorides
2 ASSAY
Procedure
Solution A: Glacial acetic acid and acetic anhydride (50:50)
Sample solution: Dissolve 350 mg of Thioridazine Hydrochloride in 80 mL of Solution A.
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant: 0.1 N perchloric acid VS
Blank: Solution A
Endpoint detection: Potentiometric
Analysis
Samples: Sample solution and Blank
Titrate the Sample solution with Titrant. Perform a blank determination, and make any necessary correction.
Calculate the percentage of thioridazine hydrochloride in the portion of Thioridazine Hydrochloride taken:
Result = {[(VS – VB) × NA × F]/W} × 100
VS = Titrant volume consumed by the Sample solution (mL)
VB = Titrant volume consumed by the Blank (mL)
NA = normality of the Titrant (mEq/mL)
F = equivalency factor, 407.04 (mg/mEq)
W = sample weight (mg)
Acceptance criteria: 99.0%–101.0% on the dried basis
3 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Change to read:
Selenium 〈291〉, Procedures, Procedure 1 (CN 1-Jun-2023)
Sample: 100 mg of sample mixed with 100 mg of magnesium oxide
Acceptance criteria: NMT 30 ppm
Organic Impurities
Diluent: Methanol and ammonium hydroxide (98:2)
Standard solution: 20.0 mg/mL of USP Thioridazine Hydrochloride RS in Diluent
Comparison solution A: 0.01 mg/mL from Standard solution in Diluent (0.05%)
Comparison solution B: 0.02 mg/mL from Standard solution in Diluent (0.1%)
Comparison solution C: 0.025 mg/mL from Standard solution in Diluent (0.125%)
Comparison solution D: 0.067 mg/mL from Standard solution in Diluent (0.33%)
Comparison solution E: 0.1 mg/mL from Standard solution in Diluent (0.5%)
Sample solution: 20 mg/mL of Thioridazine Hydrochloride in Diluent
Chromatographic system
(See Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography.)
Mode: TLC
Application volume: 5 μL
Developing solvent system: Chloroform, isopropyl alcohol, and ammonium hydroxide (74:25:1)
Spray reagent: Dragendorff's TS and Hydrogen peroxide TS
Analysis
Samples: Standard solution, Comparison solutions A–E, and Sample solution
Proceed as directed in Ordinary Impurities 〈466〉. Examine the plate under short- and long-wavelength UV light, then spray the plate with
Dragendorff's TS, dry the plate with a stream of nitrogen, and spray with hydrogen peroxide TS.
Acceptance criteria: Any secondary spot from the Sample solution is NMT 0.5%, and the sum of all secondary spots is NMT 0.5%.
4 SPECIFIC TESTS
Melting Range or Temperature 〈741〉: 159°–165°; the range between beginning and end of melting does not exceed 3°
pH 〈791〉
Sample solution: 10 mg/mL
Acceptance criteria: 4.2–5.2
Loss on Drying 〈731〉
Analysis: Dry at 105° for 4 h.
Acceptance criteria: NMT 0.4%
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
USP Reference Standards 〈11〉
USP Thioridazine Hydrochloride RS


