Thimerosal
If you find any inaccurate information, please let us know by providing your feedback here
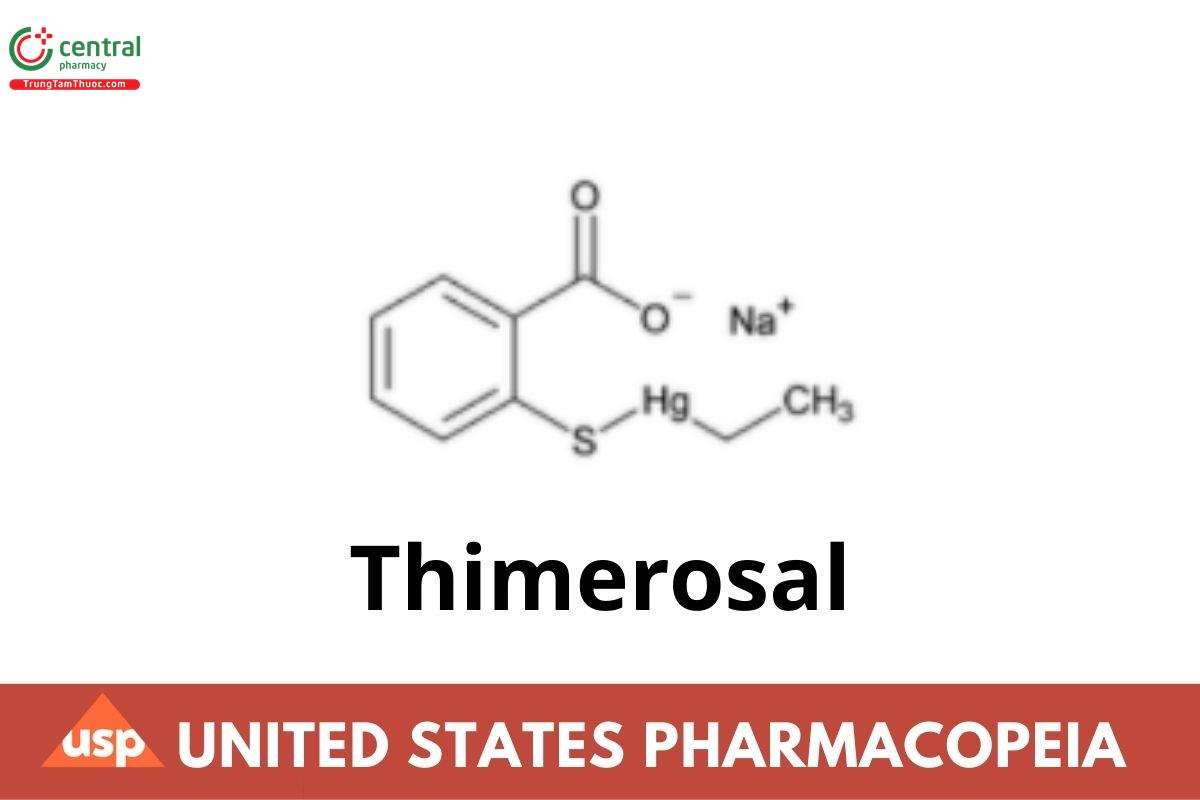
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Thimerosal contains NLT 97.0% and NMT 102.0% of thimerosal, calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B.
Sample solution: 10 mg/mL
Analysis: To the Sample solution add a few drops of silver nitrate TS.
Acceptance criteria: A pale yellow precipitate is formed.
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Solution A: 0.05% Trifluoroacetic acid, prepared by adding 1.0 mL of trifluoroacetic acid to 2 L of water
Mobile phase: Methanol and Solution A (60:40)
Standard stock solution: 250 μg/mL of USP Thimerosal RS in water
Impurity stock solution: 250 μg/mL of USP Thimerosal Related Compound A RS in methanol and water (90:10)
System suitability solution: 25 μg/mL each of USP Thimerosal RS and USP Thimerosal Related Compound A RS from Standard stock solution and Impurity stock solution, respectively, in water
Standard solution: 25 μg/mL of USP Thimerosal RS in water from Standard stock solution
Sample solution: 25 μg/mL of Thimerosal in water
[Note—Prepare both the Standard solution and Sample solution at a concentration of NMT ±10% of the specified concentration.]
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 222 nm
Column: 2.1-mm × 10-cm; 2-μm packing L1
Autosampler temperature: 4°
Flow rate: 0.35 mL/min
Injection volume: 2.5 μL
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times of thimerosal and thimerosal related compound A are 1.0 and 1.3, respectively.]
Suitability requirements
Resolution: NLT 3.5 between the thimerosal and thimerosal related compound A peaks, System suitability solution
Tailing factor: NMT 1.5, Standard solution
Relative standard deviation: NMT 0.73%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of thimerosal in the portion of Thimerosal taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Thimerosal RS in the Standard solution (μg/mL)
CU = concentration of Thimerosal in the Sample solution (μg/mL)
Acceptance criteria: 97.0%–102.0% on the dried basis
4 IMPURITIES
Mercury Ions
Iodide reagent: 332 mg/mL of potassium iodide. Prepare fresh daily. Keep the stopper in the flask, and protect from light.
Standard solution: 95 μg/mL of mercuric chloride
Sample stock solution: 5 mg/mL of Thimerosal
Sample solution A: 1 mg/mL of Thimerosal from Sample stock solution
Sample solution B: 1 mg/mL of Thimerosal from Sample stock solution and 9.5 μg/mL of mercuric chloride from Standard solution
Instrumental conditions
Mode: UV
Analytical wavelength: Determine the wavelength of maximum absorbance for the tetraiodomercurate ion at about 323 nm using the solution prepared by mixing 1.0 mL of the Standard solution and 5.0 mL of the Iodide reagent, and diluting with water to 10.0 mL.
Cell: 1 cm
Blank: Water
Analysis
Protect all solutions from light before determining their absorbances.
Samples: Label five 10-mL volumetric flasks C, D, E, F, and R. Transfer 5.0 mL of Sample solution A to flasks C and D, 5.0 mL of Sample solution B to flasks E and F, and 5.0 mL of water to
ask R. Dilute flasks C and E with water to volume. Dilute flasks D, F, and R with Iodide reagent to volume.
Determine the absorbances of the solutions in flasks C, D, E, F, and R as A , A , A , A , and A , respectively.
Calculate the percentage of mercury ions in the portion of Thimerosal taken:
Result = (AU/AS) × (CS/CU) × (Ar/Mr) × 100
AU = absorbance of the Sample solution obtained by: AU = AD − AR − AC
AS = absorbance of the Standard solution obtained by: AS = AF − AR − AE − AU
CS = concentration of mercuric chloride in Sample solution B (mg/mL)
CU = concentration of Thimerosal in Sample solution B (mg/mL)
Ar = atomic weight of mercury, 200.59
Mr = molecular weight of mercuric chloride, 271.50
Acceptance criteria: NMT 0.70%
Organic Impurities
Solution A: 0.05% Trifluoroacetic acid, prepared by adding 1.0 mL of trifluoroacetic acid to 2 L of water
Mobile phase: Methanol and Solution A (60:40)
Stock solution 1: 250 μg/mL of USP Thimerosal RS in water
Stock solution 2: 250 μg/mL of USP Thimerosal Related Compound A RS in methanol and water (90:10)
System suitability solution: 25 μg/mL each of USP Thimerosal RS and USP Thimerosal Related Compound A RS from Stock solution 1 and
Stock solution 2, respectively, in water
Standard solution: 0.25 μg/mL of USP Thimerosal Related Compound A RS from Stock solution 2 in water
Sample solution: 250 μg/mL of Thimerosal in water
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 222 nm
Column: 2.1-mm × 10-cm; 2-μm packing L1
Autosampler temperature: 4°
Flow rate: 0.35 mL/min
Injection volume: 2.5 μL
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 3.5 between the thimerosal and thimerosal related compound A peaks, System suitability solution
Relative standard deviation: NMT 3%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Thimerosal taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of thimerosal related compound A from the Standard solution
CS = concentration of USP Thimerosal Related Compound A RS in the Standard solution (μg/mL)
CU = concentration of Thimerosal in the Sample solution (μg/mL)
Acceptance criteria: See Table 1. Disregard peaks less than 0.05%.
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Thiosalicylic acida | 0.36 | 0.10 |
| Thimerosal | 1.0 | - |
Thimerosal related compound A | 1.3 | 1.0 |
Any other individual unspecified impurity | - | 0.10 |
| Total impurities | - | 1.0 |
a 2-Sulfanylbenzoic acid.
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry to constant weight under vacuum over phosphorus pentoxide.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers.
USP Reference Standards 〈11〉
USP Thimerosal RS
USP Thimerosal Related Compound A RS
2,2'-Disulfanediyldibenzoic acid.


