Technetium Tc 99m Bicisate Injection
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
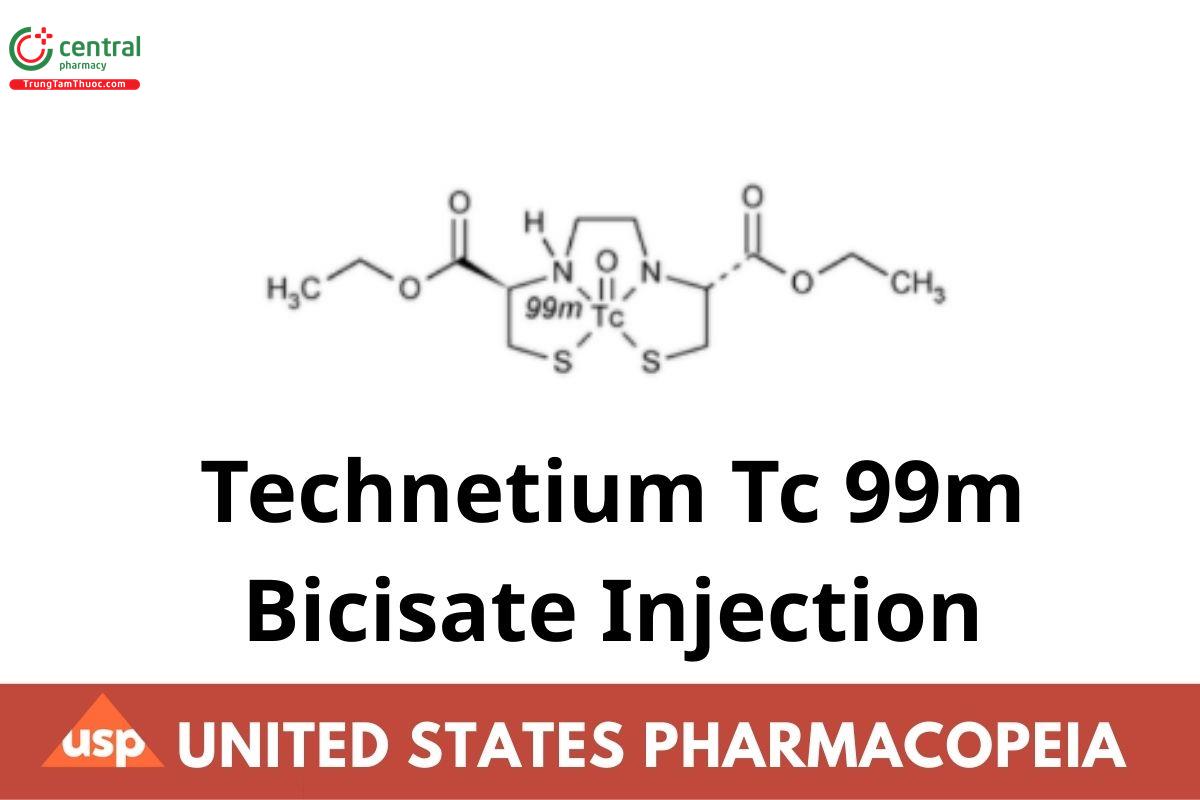
Technetium Tc 99m Bicisate Injection is a sterile, clear, colorless solution, suitable for intravenous administration, of bicisate dihydrochloride complexed to radioactive technetium (99mTc). It contains NLT 90.0% and NMT 110.0% of the labeled amount of 99mTc as a complex with bicisate, expressed in megabecquerels (or in millicuries) per milliliter at the time indicated in the labeling. Other chemical forms of radioactivity are NMT 10% of the total radioactivity.
2 IDENTIFICATION
A. Radionuclidic Identity
(See Radioactivity 〈821〉, Identification of Radionuclides.)
Acceptance criteria: Its gamma-ray spectrum is identical to that of a specimen of 99mTc that exhibits a major photopeak having an energy of 0.140 MeV.
Add the following:
B. Radiochemical Identity
Acceptance criteria: The retardation factors of the spots for Tc 99m bicisate and/or Tc(IV) 99m bicisate in the chromatogram of the Sample correspond with the ranges stated in the test for Radiochemical Impurities. (USP 1-Dec-2024)
3 ASSAY
Radioactive Concentration (Strength)
(See Radioactivity 〈821〉, Assay of Radionuclides.)
Analysis: Using a suitable counting assembly, determine the radioactivity, in megabecquerels (or millicuries) per milliliter, of the Injection by use of a calibrated system.
Acceptance criteria: 90.0%–110.0% of the labeled amount of 99mTc at the time indicated in the labeling
4 PURITY
Radionuclidic Purity
(See Radioactivity 〈821〉.)
Analysis: Using a suitable counting assembly, determine the radioactivity of each radionuclidic impurity, in kilobecquerels per megabecquerel (microcuries per millicurie) of technetium 99m, in the Injection by use of a calibrated system.
Acceptance criteria
For Injection prepared from technetium 99m derived from parent molybdenum 99 formed as a result of neutron bombardment of stable molybdenum: See Table 1.
For Injection prepared from technetium 99m derived from parent molybdenum 99 formed as a result of uranium fission-gamma-and beta-emitting impurities: See Table 2.
Table 1
| Radionuclidic Impurity | Most Prominent Photopeaks | Half-Life | Acceptance Criteria, NMTa |
| Molybdenum 99 | 0.181 MeV gamma 0.740 MeV gamma 0.780 MeV gamma | 66.0 h | 0.15 kBq/MBq (μCi/mCi) |
Total of all other gamma- emitting radionuclidic impurities | - | - | 0.5 kBq/MBq (μCi/mCi)b |
a Radioactivity of radionuclidic impurity/radioactivity of Tc 99m per administered dose of Injection at the time of administration.
b Does not exceed 92 kBq (2.5 μCi) per administered dose of the Injection at the time of administration.
Table 2
| Radionuclidic Impurity | Most Prominent/Maximum Photopeaks | Half-Life | Acceptance Criteria, NMTa |
| Molybdenum 99 | 0.181 MeV gamma 0.740 MeV gamma 0.780 MeV gamma | 66.0 h | 0.15 kBq/MBq (μCi/mCi) |
| Iodine 131 | 0.364 MeV | 8.08 d | 0.05 kBq/MBq (μCi/mCi) |
| Ruthenium 103 | 0.497 MeV | 39.5 d | 0.05 kBq/MBq (μCi/mCi) |
| Strontium 89b | 1.463 MeV beta | 52.7 d | 0.0006 kBq/MBq (μCi/mCi) |
| Strontium 90b | 0.546 MeV beta | 27.7 y | 0.00006 kBq/MBq (μCi/mCi) |
| Gross alpha impurity | - | - | 0.001 Bq/MBq (nCi/mCi) |
All other beta- and gamma- emitting radionuclidic impurities | - | - | 0.01% |
a Radioactivity of radionuclidic impurity/radioactivity of Tc 99m present at the time of administration.
b Use a counting system appropriate for the detection of particulate radiations.
Change to read:
Radiochemical Purity
Sample solution: Prepare four vials of Injection and perform the test on each vial.
Chromatographic system
(See Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 2.5-cm × 7.5-cm chromatographic silica gel sheet
Application volume: About 5 μL
Developing solvent system: Ethyl acetate
Analysis
Sample: The Sample solution used to perform this test is also used to perform the test for Radiochemical Impurities. Perform the tests in parallel with a minimal delay in spotting of the chromatographic media following the 30-min Injection incubation period. (USP 1-Dec-2024)
Place the Sample solution about 2 cm from the bottom of the Adsorbent and allow to dry for 5–10 min. Position the plate in a pre-equilibrated chromatographic chamber containing the Developing solvent system, and develop the chromatogram until the solvent front has moved 5 cm from the origin. Remove the plate from the chamber, and allow to dry. Cut the chromatographic sheet 4.5 cm from the bottom. Separately count the activity on each piece in a dose calibrator or a gamma counter. The activity on the upper portion contains the 99mTc bicisate complex, and the activity on the lower section contains all radioimpurities.
Calculate the percentage of radiochemical purity of the Injection taken:
Result = 100P/(P + C)
P = count from the top part of the sheet
C = count from the bottom part of the sheet
Acceptance criteria: NLT 90% of the total radioactivity is found as Tc99m bicisate. Calculate the mean percentage of radiochemical purity of the four test vials.
5 IMPURITIES
Change to read:
Radiochemical Impurities
(USP 1-Dec-2024)
Chromatographic system
(See Chromatography 〈621〉, General Procedures, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 2.5-cm × 7.5-cm reverse-phase thin-layer chromatographic plate (or equivalent)
Developing solvent system: Acetone and 0.5 M ammonium acetate (60:40)
Application volume: About 2 μL
Analysis
Sample: Sample solution used to perform the test for Radiochemical Purity. Perform the tests in parallel with a minimal delay in spotting of the chromatographic media following the 30-min Injection incubation period.
Apply the Sample 1 cm from the bottom of the Adsorbent, and allow the spot to air-dry thoroughly. Develop the chromatogram until the solvent front has moved 7 cm from the origin. Remove the plate from the chamber and air-dry. Using a suitable calibrated scanner, determine the compounds present by calculating the retention factors for all peaks present. Compounds and approximate R values are shown in Table 3.
Table 3
| Compound | Approximate RF Value |
| 99mTc bicisate | 0.15–0.44 |
| 99mTc(IV) bicisate | 0.3–0.4 |
| 99mTc bicisate and 99mTc(IV) bicisate | 0.15–0.44 |
| Hydrolyzed reduced Tc | 0.00–0.14 |
| Free pertechnetate and 99mTc ethylene cisteinate monomer | 0.70–0.84 |
| 99mTc EDTA | 0.95–1.0 |
Calculate the quantity of 99mTc(IV) ligand in the Injection by subtracting the 99mTc bicisate percentage obtained in the test for Radiochemical Purity from the combined 99mTc bicisate and 99mTc(IV) bicisate area percentage obtained in the test for Radiochemical Impurities.
Acceptance criteria: The sum of the impurities is NMT 10%.
6 SPECIFIC TESTS
Add the following:
Appearance: Clear, colorless solution, free from visible particulates (USP 1-Dec-2024)
Change to read:
Bacterial Endotoxins Test 〈85〉: Meets the requirements. The Injection may be distributed or dispensed prior to completion of the test. (USP 1-Dec-2024)
Add the following:
Sterility Tests 〈71〉: Meets the requirements. The Injection may be distributed or dispensed prior to completion of the test. (USP 1-Dec-2024)
Delete the following:
Other Requirements (USP 1-Dec-2024)
7 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in single-dose or multiple-dose containers, at controlled room temperature.
Labeling: Label the Injection to include the following, in addition to the information specified under Labeling 〈7〉, Labels and Labeling for
Injectable Products: the time and date of calibration; the amount of 99mTc as labeled bicisate expressed as total megabecquerels (or millicuries) per milliliter at the time of calibration; the expiration date and time; the lot number; and the statement: [Caution—Radioactive Material]. The labeling indicates that, in making dosage calculations, correction is to be made for radioactive decay, and also indicates that the radioactive half-life of 99mTc is 6.0 h.


