Tazobactam
If you find any inaccurate information, please let us know by providing your feedback here
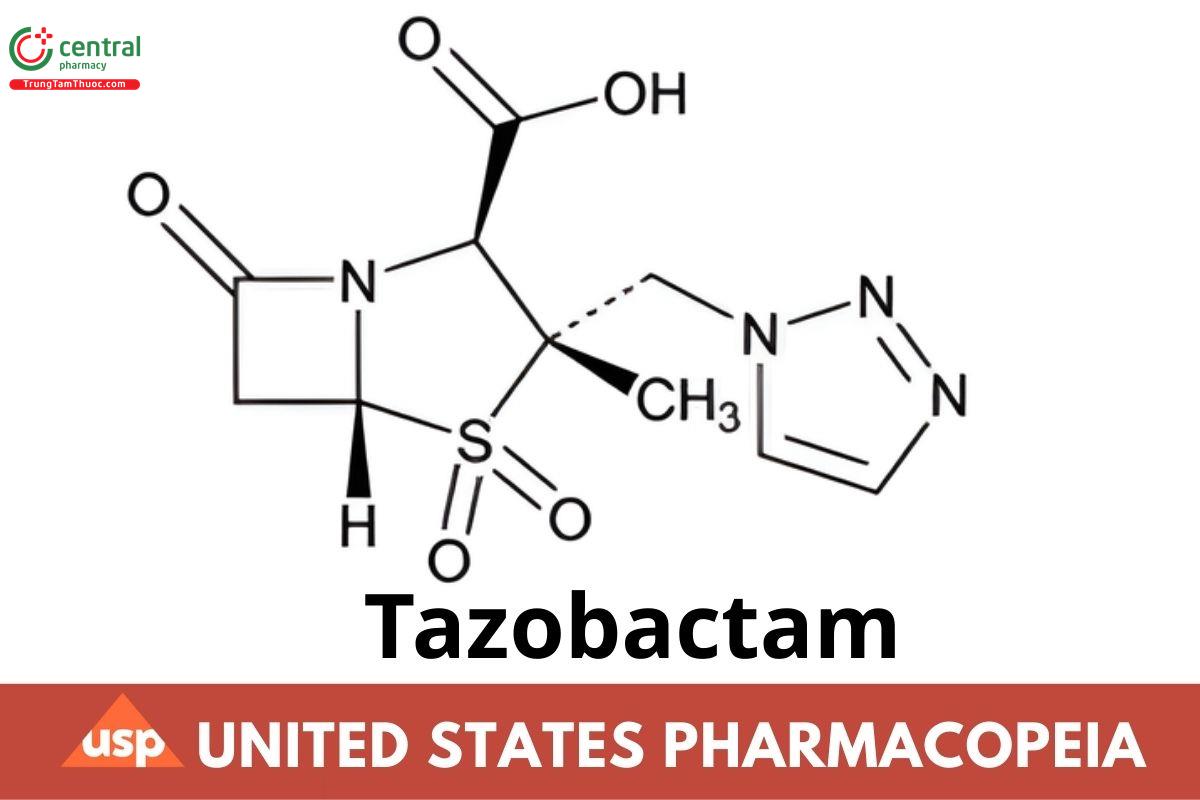
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C10H12N4O5S 300.29
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-, 4,4-dioxide, [2S-(2α,3β,5α)]-;
(2S,3S,5R)-3-Methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 4,4-dioxide CAS RN®: 89786-04-
9; UNII: SE10G96M8W.
C10H12N4O5S · 1⁄2H2O 309.30 CAS RN®: 428863-55-2.
1 DEFINITION
Tazobactam contains NLT 98.0% and NMT 102.0% of C10H12N4O5S, calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Mobile phase: Dissolve 1.32 g of dibasic ammonium phosphate in 750 mL of water. Adjust with 5% v/v phosphoric acid to a pH of 2.5, and dilute with water to 1000 mL. Add 30 mL of acetonitrile, mix, and pass through a filter of 0.2-μm pore size.
System suitability solution: 16 μg/mL of l-phenylalanine, 50 μg/mL of USP Tazobactam RS, and 8 μg/mL of USP Tazobactam Related
Compound A RS in Mobile phase. Maintain the System suitability solution at 3° until injection. Prepare fresh daily. If an autosampler is used, replace the plastic tubing connected to the injection needle with a stainless steel assembly, and maintain at 3°. If a chilled autosampler is not used, then this solution should be injected immediately after preparation.
Standard solution: 0.5 mg/mL of USP Tazobactam RS in Mobile phase. Cool, and maintain the Standard solution at 3° until injection. If an autosampler is used, replace the plastic tubing connected to the injection needle with a stainless steel assembly, and maintain at 3°. If a chilled autosampler is not used, then this solution should be injected immediately after preparation.
Sample solution: 0.5 mg/mL of Tazobactam in Mobile phase. Cool, and maintain the Sample solution at 3° until injection. If an autosampler is used, replace the plastic tubing connected to the injection needle with a stainless steel assembly, and maintain at 3°. If a chilled autosampler is not used, then this solution should be injected immediately after preparation.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 210 nm
Column: 4.6-mm × 25-cm; 5-μm packing L1
Flow rate: 1.5 mL/min
Injection size: 20 μL
System suitability
Samples: System suitability solution and Standard solution
[Note—See Table 1 for the relative retention times.]
Suitability requirements
Resolution: NLT 6.0 between tazobactam and l-phenylalanine, System suitability solution
Tailing factor: NMT 1.8, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of tazobactam (C10H12N4O5S) in the portion of Tazobactam taken:
Result = (rU/rS ) × (CS /CU ) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Tazobactam RS in the Standard solution (mg/mL)
CU = concentration of the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Organic Impurities
Mobile phase, System suitability solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Blank: Mobile phase. Cool, and maintain the Blank at 3° until injection. If an autosampler is used, replace the plastic tubing connected to the injection needle with a stainless steel assembly, and maintain at 3°. If a chilled autosampler is not used, then this solution should be injected immediately after preparation.
Sample solution: Prepare as directed in the Assay. Cool, and maintain the Sample solution at 3° until injection. If an autosampler is used, replace the plastic tubing connected to the injection needle with a stainless steel assembly, and maintain at 3°. If a chilled autosampler is not used, then this solution should be injected immediately after preparation.
Analysis
Samples: Blank and Sample solution
Ignore any peaks of the Sample solution that correspond to any peaks of the Blank.
Calculate the percentage of each impurity in the portion of Tazobactam taken:
Result = (rU/rT ) × 100
rU = peak response for each impurity in the Sample solution
rT = sum of all the peak responses in the Sample solution
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
|---|---|---|
| Tazobactam related compound A | 0.29 | 1.0 |
| L-Phenylalanine | 0.71 | – |
| Tazobactam | 1.0 | – |
| Any other individual impurity | – | 0.1 |
| Total impuritiesa | – | 0.3 |
a Total of all impurities other than tazobactam related compound A.
5 SPECIFIC TESTS
Bacterial Endotoxins Test 〈85〉: The level of bacterial endotoxins is such that the requirements of the relevant dosage form monograph(s) in which Tazobactam is used can be met.
Optical Rotation, Specific Rotation〈781S〉
Sample solution: 10 mg/mL, in dimethylformamide
Acceptance criteria: +160° to +167° (t = 20°)
Microbial Enumeration Tests 〈61〉and Tests for Specified Microorganisms 〈62〉: The total aerobic microbial count does not exceed 103 cfu/g, and the total combined molds and yeasts count does not exceed 102 cfu/g.
pH 〈791〉
Sample solution: 2.5 mg/mL
Acceptance criteria: 1.8–2.8
Water Determination, Method I〈921〉: NMT 0.6% for the anhydrous form; 2.2–3.8% for the hemihydrate form
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at controlled room temperature.
Labeling: Where it is the hemihydrate form, the label so indicates.
USP Reference Standards 〈11〉
USP Tazobactam RS
USP Tazobactam Related Compound A RS
(2S,3S)-2-Amino-3-methyl-3-sulfino-4-(1H-1,2,3-triazol-1-yl)butyric acid.
C7H12N4O4S 248.26


