Tadalafil
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
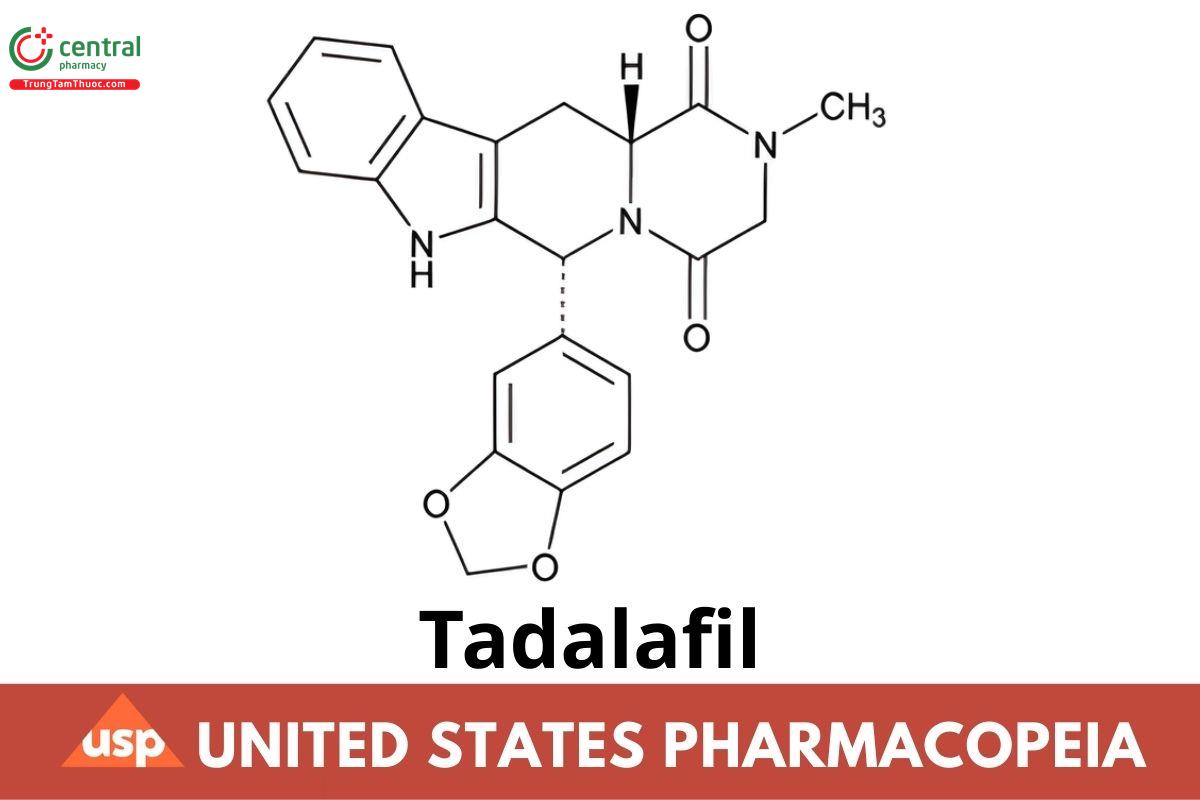
C22H19N3O4 389.40
Pyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-, (6R-12aR)-;
(6R,12aR)-2,3,6,7,12,12a-Hexahydro-2-methyl-6-[3,4-(methylenedioxy)phenyl] pyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione CAS RN®: 171596-
29-5; UNII: 742SXX0ICT.
1 DEFINITION
Tadalafil contains NLT 97.5% and NMT 102.5% of Tadalafil (C22H19N3O4 ), calculated on the dried basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the Identi
cation solution, as obtained in the test for
Enantiomeric and Diastereomeric Purity.
3 ASSAY
3.1 Procedure
Solution A: Add 1.0 mL of tri
uoroacetic acid to 1 L of water.
Mobile phase: Acetonitrile and Solution A (45:55)
Standard solution: 0.1 mg/mL of USP Tadalafil RS in acetonitrile and Solution A (1:1); prepare by first dissolving the standard in acetonitrile, and then diluting with Solution A to final volume.
Sample solution: 0.1 mg/mL of Tadalafil in acetonitrile and Solution A (1:1); prepare by first dissolving the sample in acetonitrile, and then diluting with Solution A to final volume.
3.2 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: 285 nm
Column: 4.6-mm × 25-cm; 5-μm packing L7
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 20 μL
3.3 System suitability
Sample: Standard solution
3.4 Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 0.73%
3.5 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of Tadalafil (C22H19N3O4 ) in the portion of Tadalafil taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Tadalafil RS in the Standard solution (mg/mL)
CU = concentration of Tadalafil in the Sample solution (mg/mL)
Acceptance criteria: 97.5%–102.5% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.10%, using a 1-g sample
4.1 Organic Impurities
[Note—Do not use sonication during the preparation of analyte solutions.]
Solution A: Add 1.0 mL of trifluoroacetic acid to 1 L of water.
Solution B: Acetonitrile
Mobile phase: See Table 1. Return to original conditions and re-equilibrate the column.
Table 1
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 85 | 15 |
| 3 | 85 | 15 |
| 30 | 5 | 95 |
| 33 | 5 | 95 |
Standard solution: 0.4 μg/mL of USP Tadalafil
RS in acetonitrile and Solution A (1:1); prepare by first dissolving the standard in acetonitrile, and then diluting with Solution A to final volume.
Sensitivity solution: 0.2 μg/mL of USP Tadalafil
RS in acetonitrile and Solution A (1:1) from the Standard solution
System suitability stock solution: To generate the 6R,12aS diastereomer of Tadalafil, dissolve 4.0 mg of Tadalafil in 50 mL of a mixture of isopropyl alcohol and acetonitrile (1:1). Add 1.0 mL of 1.0 M tetrabutylammonium hydroxide in methanol, and allow to stand at room temperature for 40 min. Add 1.0 mL of tri fluoroacetic acid, and dilute with a mixture of isopropyl alcohol and acetonitrile (1:1) to 100 mL.
System suitability solution: Dissolve 40 mg of Tadalafil in 50 mL of acetonitrile. Add 2.0 mL of the System suitability stock solution, and dilute with Solution A to 100 mL.
Sample solution: 0.4 mg/mL of Tadalafil in acetonitrile and Solution A (1:1); prepare by first dissolving the sample in acetonitrile, and then diluting with Solution A to final volume.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 285 nm
Column: 4.6-mm × 25-cm; 5-μm packing L7
Column temperature: 40°
Flow rate: 1.0 mL/min
Injection volume: 20 μL
System suitability
Samples: Standard solution, Sensitivity solution, and System suitability solution
[Note—The relative retention times for Tadalafil and the 6R,12aS diastereomer of Tadalafil are about 1.0 and 1.03, respectively.]
Suitability requirements
Tailing factor: NMT 1.5, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution
Peak-to-valley ratio: The ratio of the height of the 6R,12aS diastereomer peak to the height of the valley between the 6R,12aS diastereomer peak and Tadalafil is NLT 3.3, System suitability solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Tadalafil taken:
Result = (rU/rS) × (CS/CU ) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of Tadalafil from the Standard solution
CS = concentration of USP Tadalafil RS in the Standard solution (mg/mL)
CU = concentration of Tadalafil in the Sample solution (mg/mL)
4.2 Acceptance criteria
[Note—Disregard peaks due to the 6R,12aS and 6S,12aR diastereomers of Tadalafil, which co-elute at a retention time of about 1.03 relative to Tadalafil. The diastereomers are controlled in the test for Enantiomeric and Diastereomeric Purity.]
Individual impurities: NMT 0.1%
Total impurities: NMT 0.3%
Reporting level for impurities: 0.05%
Enantiomeric and Diastereomeric Purity
Mobile phase: Hexanes and isopropyl alcohol (50:50)
Diluent: Hexanes, isopropyl alcohol, and acetonitrile (40:40:20)
Identification solution: 0.5 mg/mL of USP Tadalafil
RS in Diluent. [Note—This solution is used for Identification test B.]
Standard stock solution: 50 μg/mL of USP Tadalafil RS in Diluent
Standard solution: 0.5 μg/mL of USP Tadalafil RS in Diluent from the Standard stock solution
System suitability stock solution: To generate the 6R,12aS diastereomer of Tadalafil, dissolve 25 mg of Tadalafil in 40 mL of Diluent. Add 1.0 mL of 1.0 M tetrabutylammonium hydroxide in methanol, and allow to stand at room temperature for 20 min. Add 1.0 mL of trifluoroacetic acid, and dilute with Diluent to 50 mL.
System suitability solution: Transfer 1.0 mL of the System suitability stock solution and 10 mL of the Standard stock solution to a 50-mL volumetric flask, and dilute with Diluent to volume.
Sensitivity solution: 0.25 μg/mL of USP Tadalafil RS in Diluent from Standard solution
Sample solution: 0.5 mg/mL of Tadalafil in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: 222 nm
Column: 4.6-mm × 25-cm; 10-μm packing L51
Column temperature: 30°
Flow rate: 0.75 mL/min
Injection volume: 10 μL
System suitability
Samples: Standard solution, System suitability solution, and Sensitivity solution
Suitability requirements
Resolution: NLT 2.0, between the 6R,12aS diastereomer and Tadalafil, System suitability solution
Tailing factor: NLT 0.8 and NMT 1.5, Standard solution
Relative standard deviation: NMT 10.0%, Standard solution
Signal-to-noise ratio: NLT 20, Sensitivity solution
Analysis
Samples: Identification solution, Standard solution, and Sample solution
Calculate the percentage of each stereoisomer impurity in the portion of Tadalafil taken:
Result = (rU/rS) × (CS/CU ) × 100
rU = peak response of each stereoisomer impurity from the Sample solution
rS = peak response of Tadalafil from the Standard solution
CS = concentration of USP Tadalafil RS in the Standard solution (mg/mL)
CU = concentration of Tadalafil in the Sample solution (mg/mL)
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
|---|---|---|
| 6R,12aS diastereomera | 0.79 | 0.1 |
| Tadalafil | 1.0 | – |
| 6S,12aS enantiomerb | 1.4 | 0.1 |
| 6S,12aR diastereomerc | 1.7 | 0.1 |
a (6R,12aS)-6-(1,3-Benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-pyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione.
b (6S,12aS)-6-(1,3-Benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-pyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione.
c (6S,12aR)-6-(1,3-Benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-pyrazino[1′,2′:1,6]pyrido[3,4-b]indole-1,4-dione.
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry a sample under vacuum at 105° for 3 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers. Store at room temperature.
USP Reference Standards 〈11〉
USP Tadalafil RS


