Tacrolimus
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
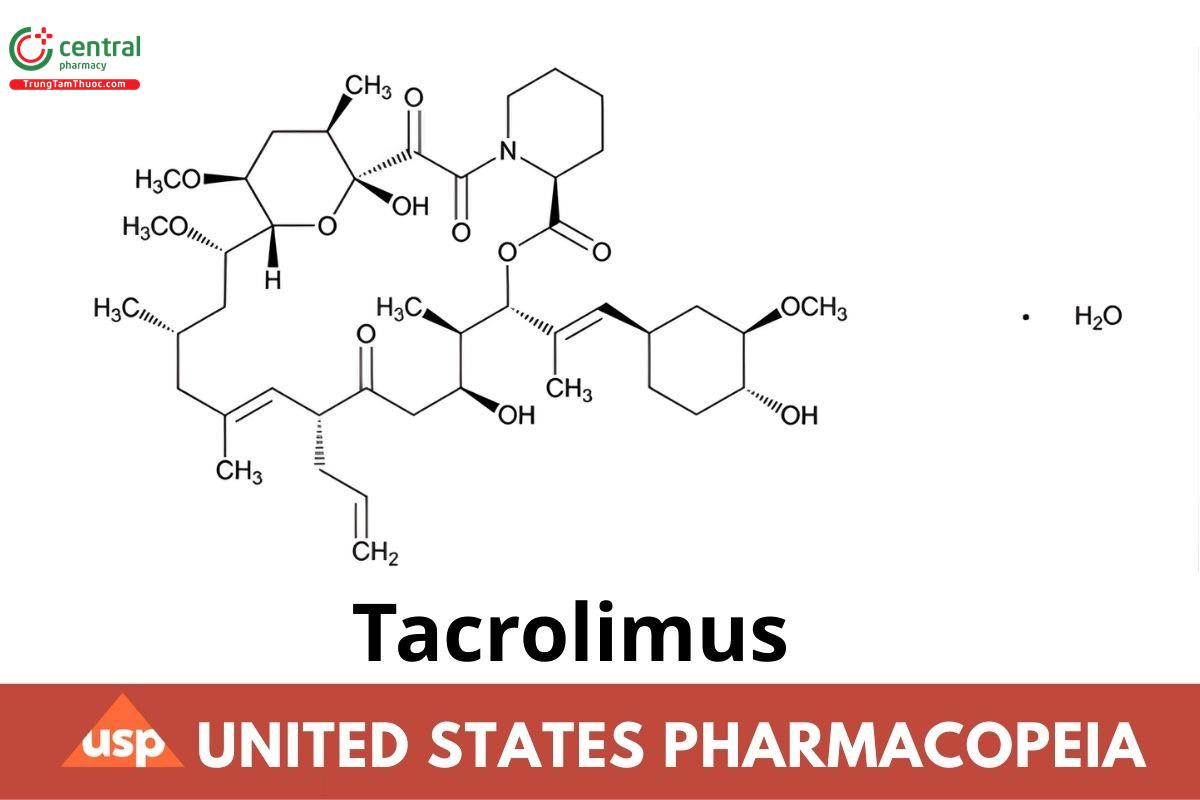
C44H69NO12· H2 O 822.03
15,19-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro- 5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-, monohydrate, [3S-[3R*,E(1S*,3S*,4S*)],4S*,5R*,8S*,9E,12R*,14R*,15S*,16R*,18S*,19S*,26aR*]]-;
(−)-(3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-8-Allyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[(E)-2- [(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone monohydrate CAS RN®: 109581-93-3; UNII: WM0HAQ4WNM.
1 DEFINITION
Tacrolimus contains NLT 98.0% and NMT 102.0% of Tacrolimus (C44H69NO12), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution as obtained in the Assay.
3 ASSAY
3.1 Procedure
Protect solutions containing tacrolimus from light.
Solution A: 6 mM phosphoric acid
Solution B: Acetonitrile and tert-butyl methyl ether (81:19)
Solution C: Solution A and Solution B (4:1)
Solution D: Solution A and Solution B (1:4)
Mobile phase: See Table 1.
Table 1
| Time (min) | Solution C (%) | Solution D (%) |
|---|---|---|
| 0 | 72 | 28 |
| 30 | 72 | 28 |
| 53 | 15 | 85 |
| 54 | 72 | 28 |
| 60 | 72 | 28 |
Diluent: Acetonitrile and water (7:3)
System suitability solution: 3 mg/mL of USP Tacrolimus System Suitability Mixture RS in Diluent. Allow the solution to stand for 3 h at ambient temperature before use.
Standard solution: 3 mg/mL of USP Tacrolimus RS in Diluent. Allow the solution to stand for 3 h at ambient temperature before use.
Sample solution: 3 mg/mL of Tacrolimus in Diluent. Allow the solution to stand for 3 h at ambient temperature before use.
3.2 Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 15-cm; 3-μm packing L1
Temperatures
Column: 60°
Autosampler: 4°
Flow rate: 1.5 mL/min
Injection volume: 20 μL
3.3 System suitability
Samples: System suitability solution and Standard solution
[Note—See Table 3 for relative retention times.]
3.4 Suitability requirements
Resolution: NLT 3.0 between ascomycin and tacrolimus, System suitability solution
Relative standard deviation: NMT 1.0% for the sum of the responses of tacrolimus, tacrolimus open ring, and tacrolimus 19-epimer,
Standard solution
3.5 Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of tacrolimus (C44H69NO12) in the portion of Tacrolimus taken:
Result = (rU/rS) × (CS/CU) × 100
rU = sum of the peak responses of tacrolimus open ring, tacrolimus 19-epimer, and tacrolimus from the Sample solution
rS = sum of the peak responses of tacrolimus open ring, tacrolimus 19-epimer, and tacrolimus from the Standard solution
CS = concentration of USP Tacrolimus RS in the Standard solution (mg/mL)
CU = concentration of Tacrolimus in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous and solvent-free basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Organic Impurities, Procedure 1
Use Organic Impurities, Procedure 1 when the impurity prodfie includes tacrolimus methylacrylaldehyde and tacrolimus diene. It is suggested that new columns be conditioned with about 500 mL of alcohol before use to meet the resolution criterion.
Mobile phase: Hexane, n-butyl chloride, and acetonitrile (7:2:1). Add n-butyl chloride to hexane, and mix well before adding acetonitrile. After adding acetonitrile, mix the Mobile phase for 2 h to get a clear solution. Any deviations from the ratio of components in the Mobile phase and the order of mixing will result in a two-phase solution.
System suitability solution: 0.1 mg/mL each of USP Tacrolimus RS and USP Tacrolimus Related Compound A RS in Mobile phase
Sample solution: 2.0 mg/mL of Tacrolimus in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 225 nm
Column: Two 4.6-mm × 25-cm columns; 5-μm packing L20
Column temperature: 28 ± 2°
Flow rate: 1.5 mL/min. Adjust the Flow rate so that the retention time of tacrolimus is approximately 15 min.
Injection volume: 20 μL
System suitability
Sample: System suitability solution
Suitability requirements
Resolution: NLT 1.1 between tacrolimus and tacrolimus related compound A
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
Analysis
Sample: Sample solution
Calculate the percentage of each impurity in the portion of Tacrolimus taken:
Result = (rU /Fi ) × {1/[rT + Σ(rU /Fi )]} × 100
rU = peak response of each impurity from the Sample solution
Fi = relative response factor for each corresponding impurity (see Table 2)
rT = peak response of tacrolimus from the Sample solution
Acceptance criteria: See Table 2.
Table 2
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Tacrolimus meth ylacryl aldehydea | 0.55 | 16.7 | 0.2 |
| Tacrolimus dieneb | 0.79 | 2.2 | 0.2 |
| Tacrolimus impurity 1c | 0.96 | 1.0 | 0.2 |
| Tacrolimus related compound Ad | 0.96 | – | – |
| Tacrolimus | 1.0 | 1.0 | – |
| Tacrolimus 19-epimerd,e | 1.1 | – | – |
| Tacrolimus open ringd,f | 1.3 | – | – |
| Any individual unspecified impurity | – | 1.0 | 0.2 |
| Total impuritiesg | – | – | 0.3 |
a (E)-3-[[(1R,3R,4R)-4-Hydroxy-3-methoxycyclohexyl]-2-methylacrylaldehyde.
b (14E,18E)-17-Allyl-1-hydroxy-12-[(E)-2-(4-hydroxy-3-methoxycyclohexyl)-1-methylvinyl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28- dioxa-4-azatricyclo[22.3.1.04,9] octacosa-14,18-diene-2,3,10,16-tetrone.
c Specified unidentified impurity.
d For informational purposes only; not to be reported.
e (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19S,26aS)-8-Allyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a,hexadecahydro-5,19-dihydroxy-3- {(E)-2-[1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl}-14,16,dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone.
f (3S,4R,5S,8R,12S,14S,15R,16S,18R,26aS,E)-8-Allyl-5,6,11,12,13,14,15,16,17,18,24,25,26,26a-tetradecahydro-5,15,20,20-tetrahydroxy-3- {(E)-2-[1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl}-14,16-dimethoxy-4,10,12,18-tetramethyl-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,19,21(4H,8H,20H,23H)-tetrone.
g Total impurities limit does not include tacrolimus open ring and tacrolimus 19-epimer.
Organic Impurities, Procedure 2
Use Organic Impurities, Procedure 2 when the impurity profile includes ascomycin, desmethyl tacrolimus, tacrolimus 8-epimer, and tacrolimus 8- propyl analog. Protect solutions containing tacrolimus from light.
Solution A, Solution B, Solution C, Solution D, Mobile phase, Diluent, System suitability solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Standard solution: 30 μg/mL of USP Tacrolimus RS in Diluent. Allow the solution to stand for 3 h at ambient temperature before use.
Reporting threshold solution: 1.5 μg/mL of USP Tacrolimus RS in Diluent
Peak identification solution 1: 10 μg/mL of USP Tacrolimus 8-epimer RS in acetonitrile
Peak identification solution 2: 10 μg/mL of USP Tacrolimus 8-propyl Analog RS in acetonitrile
System suitability
[Note—Identify the related compounds by the relative retention times provided in Table 3.]
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 3.0 between tacrolimus and ascomycin, System suitability solution
Relative standard deviation: NMT 10.0% for the sum of the responses of tacrolimus and tacrolimus 19-epimer, Standard solution
Analysis
Samples: Sample solution, Standard solution, Reporting threshold solution, Peak identification solution 1, and Peak identification solution 2
Calculate the percentage of each impurity in the portion of Tacrolimus taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of each impurity from the Sample solution
rS = sum of the peak responses of tacrolimus 19-epimer and tacrolimus from the Standard solution
CS = concentration of USP Tacrolimus RS in the Standard solution (mg/mL)
CU = concentration of Tacrolimus in the Sample solution (mg/mL)
Acceptance criteria: See Table 3. Identify tacrolimus 8-epimer and tacrolimus 8-propyl analog using Peak identification solution 1 and Peak identification solution 2. Report impurity peaks with responses NLT that of the peak in the Reporting threshold solution (0.05%). Disregard peaks with retention times less than 3 min.
Table 3
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
|---|---|---|
| Tacrolimus open ringa,b | 0.52 | – |
| Ascomycin 19-epimer (if present)c,d | 0.54 | 0.1 |
| Tacrolimus 19-epimerb,e | 0.63 | – |
| Ascomycinf | 0.87 | 0.50 |
| Desmethyl tacrolimus (if present)d,g | 0.94 | 0.1 |
| Tacrolimus | 1.00 | – |
| Tacrolimus 8-epimerh | 1.28 | 0.15 |
| Tacrolimus 8-propyl analogi | 1.33 | 0.15 |
| Any individual unspecified impurity | – | 0.1 |
| Total impuritiesj | – | 1.0 |
a (3S,4R,5S,8R,12S,14S,15R,16S,18R,26aS,E)-8-Allyl-5,6,11,12,13,14,15,16,17,18,24,25,26,26a-tetradecahydro-5,15,20,20-tetrahydroxy-3- {(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl}-14,16-dimethoxy-4,10,12,18-tetramethyl-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,19,21(4H,8H,20H,23H)-tetrone.
b Tacrolimus open ring and tacrolimus 19-epimer are isomers of tacrolimus, which are present in equilibrium with the active ingredient. They are not to be reported as degradation products.
c (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19S,26aS)-8-Ethyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3- [(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21-(4H,23H)-tetrone.
d If possible from the manufacturing process.
e (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19S,26aS)-8-Allyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3- {(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl}-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone.
f (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-8-Ethyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3- [(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21-(4H,23H)-tetrone.
g (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-8-Allyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3- [(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl]-14,16-dimethoxy-4,12,18-trimethyl-15,19-epoxy-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21-(4H,23H)-tetrone.
h (3S,4R,5S,8S,9E,12S,14S,15R,16S,18R,19R,26aS)-8-Allyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3- {(E)-2-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl}-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone.
i (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)- 5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-Hexadecahydro-5,19-dihydroxy-3-{(E)-2- [(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl}-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-8-propyl-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone.
j Total impurities limit does not include tacrolimus open ring and tacrolimus 19-epimer.
5 SPECIFIC TESTS
Optical Rotation, Specific Rotation 〈781S〉
Sample solution: 10 mg/mL in N,N-dimethylformamide
Acceptance criteria: −110° to −115° on the “as-is” basis
Water Determination, Method I 〈921〉: NMT 4.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store at controlled room temperature.
Labeling: If a test for Organic Impurities other than Procedure 1 is used, then the labeling states with which test for Organic Impurities the article complies.
Change to read:
USP Reference Standards 〈11〉
USP Tacrolimus RS
15,19-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone-5,6,8,11,12,13,14,15, 16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-, monohydrate, [3S-[3R*,E(1S*,3S*,4S*)],4S*,5R*,8S*,9E,12R*,14R*,15S*,16R*, 18S*,19S*,26aR*]]-.
C44H69NO12 · H2O 822.03
USP Tacrolimus Related Compound A RS
(E)-8-Ethyl-5,6,8,11,12,13,14,15,16,17,18,19,24, 25,26,26a-Hexadecahydro-5,19-dihydroxy-3-[(E)-2-(4-hydroxy-3-methoxycyclohexyl)-1- methylvinyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21-(4H,23H)-tetrone.
C43H69NO12 792.02 (ERR 1-Sep-2021)
USP Tacrolimus 8-epimer RS
(3S,4R,5S,8S,9E,12S,14S,15R,16S,18R,19R,26aS)-8-Allyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-{(E)-2- [(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl}-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone.
C44H69NO12 804.03 (ERR 1-Sep-2021)
USP Tacrolimus 8-propyl Analog RS
(3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-Hexadecahydro-5,19-dihydroxy-3-{(E)-2- [(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl}-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-8-propyl-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone.
C44H71NO12 806.03
USP Tacrolimus System Suitability Mixture RS
This is a mixture of tacrolimus, ascomycin
(3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-8-Ethyl-5,6,8,11,12,13,14,15,16,17,18,19,24, 25,26,26a-hexadecahydro-5,19-dihydroxy-3-[(E)-2- [(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21-(4H,23H)-tetrone.
C43H69NO12 792.01
and tacrolimus 8-propyl analog
(3S,4R,5S,8R,9E,12S,14S,15R,16S,18R, 19R,26aS)-5,6,8,11,12,13,14,15,16,17,18,19,24, 25,26,26a-Hexadecahydro-5,19-dihydroxy-3-{(E)-2- [(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylvinyl}-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-8-propyl-3H-pyrido[2,1-c] [1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone.
C44H71NO12 806.03


