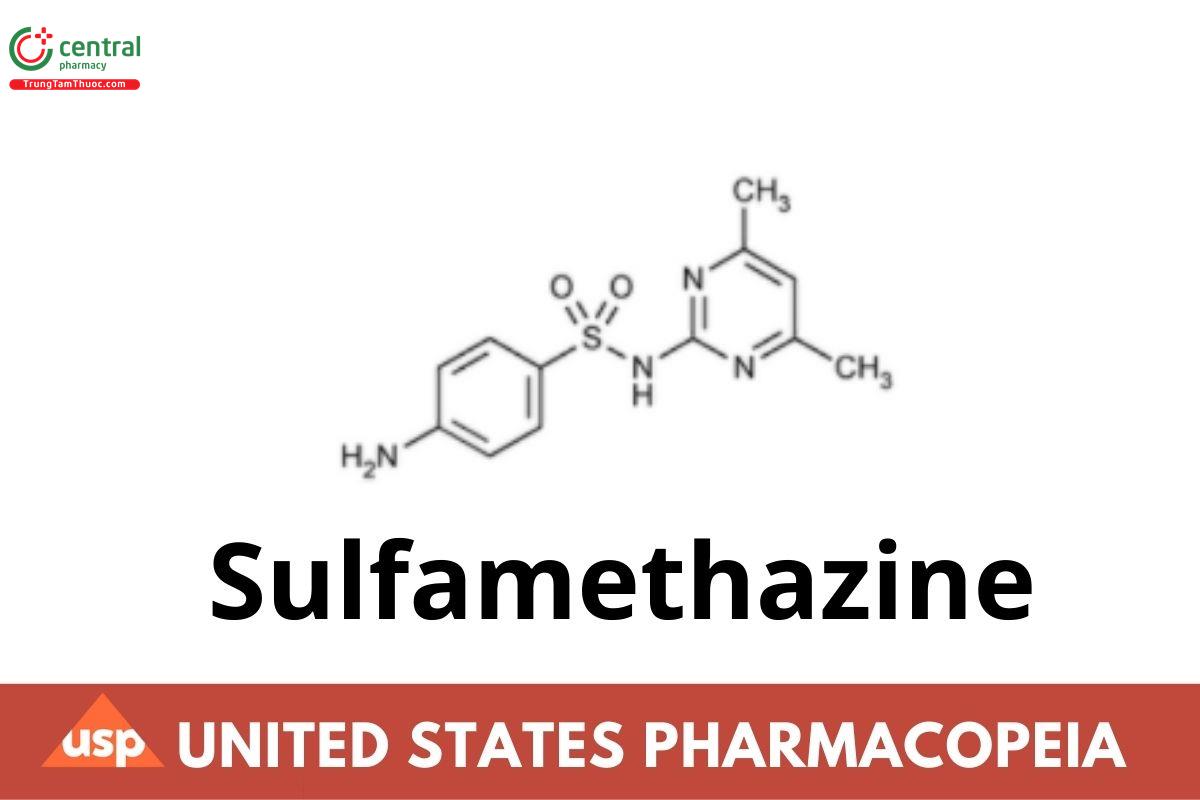
Sulfamethazine
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Sulfamethazine contains NLT 99.0% and NMT 100.5% of sulfamethazine (C12H14N4O2S), calculated on the dried basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. Procedure
Sample: 0.10 g of Sulfamethazine
Analysis: To the Sample add 10 mL of water and just sufficient 0.1 N sodium hydroxide (4 mg/mL) to give a faint pink spot on phenolphthalein paper. Add 5 drops of cupric sulfate TS.
Acceptance criteria: A yellow-green precipitate is formed, and it becomes brown on standing.
3 ASSAY
Procedure
Analysis: Proceed as directed in Nitrite Titration 〈451〉.
Calculate the percentage of sulfamethazine (C12H14N4O2S) in the portion of Sulfamethazine taken:
Result = V × M × F × (1/W) × 100
V = volume of Titrant consumed by the Sample (mL)
M = actual Titrant concentration, in molarity (mEq/mL)
F = equivalency factor of sulfamethazine, 278.3 mg/mEq
W = weight of the Sample taken (mg)
Acceptance criteria: 99.0%–100.5% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Delete the following:
Selenium 〈291〉:
Sample: 200 mg of Sulfamethazine
Acceptance criteria: NMT 30 ppm (USP 1-Aug-2022)
Ordinary Impurities 〈466〉
Sample solution: Acetone
Standard solution: Acetone
Eluant: Ethyl acetate, methanol, and ammonium hydroxide (17:6:5)
Visualization: 11
5 SPECIFIC TESTS
Melting Range or Temperature 〈741〉: 197°–200°
Acidity
Sample: 3.0 g of Sulfamethazine
Sample solution: Digest the Sample with 150 mL of carbon dioxide-free water at 70° for about 5 min, stirring occasionally to maintain suspension of the Sample. Cool the mixture rapidly in an ice bath to 20 ± 0.5°, with mechanical stirring. Filter immediately, with vacuum, omitting washing of the solid but drying it thoroughly by suction. Use the filtrate.
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant 1: 0.1 N sodium hydroxide VS
Titrant 2: 0.1 M sodium nitrite VS
Endpoint detection: Visual
Analysis 1: To 25.0 mL of the Sample solution add 2 drops of thymolphthalein TS. Titrate with Titrant 1. Record the volume of Titrant 1 consumed (V ).
Analysis 2: To a second 25.0-mL portion of the Sample solution add 10 mL of hydrochloric acid. Cool the mixture to 15°, and proceed as directed in Nitrite Titration 〈451〉. Record the volume of Titrant 2 consumed (V ).
Result = V1 − V2
V1 = volume of Titrant 1 consumed by the Sample solution in Analysis 1 (mL)
V2 = volume of Titrant 2 consumed by the Sample solution in Analysis 2 (mL)
Acceptance criteria: NMT 0.5 mL
Loss on Drying 〈731〉
Sample: 1 g of Sulfamethazine
Analysis: Dry at 105° for 2 h.
Acceptance criteria: NMT 0.5%
Clarity and Color of Solution
Sample: 1 g of Sulfamethazine
Analysis: Dissolve the Sample in a mixture of 20 mL of water and 5 mL of 1 N sodium hydroxide.
Acceptance criteria: The solution is clear and not more deeply colored than pale yellow.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed, light-resistant containers.
USP Reference Standards 〈11〉
USP Sulfamethazine RS


