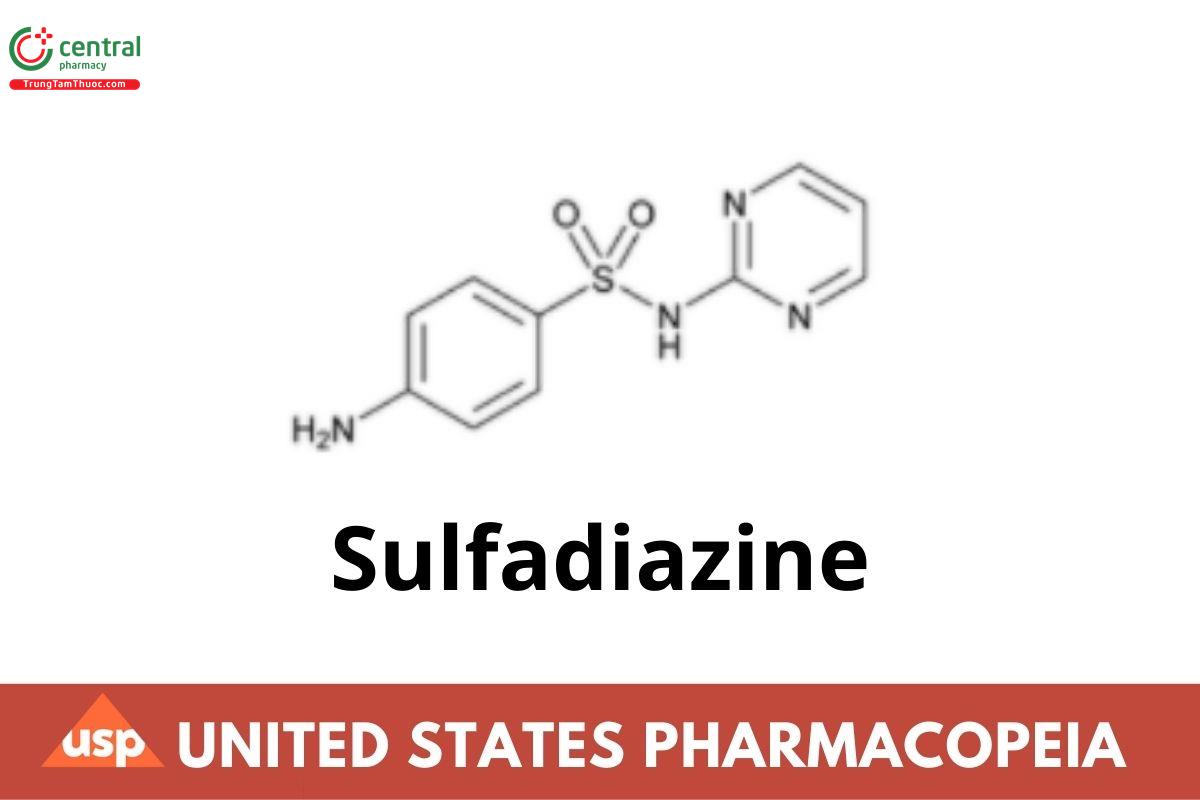
Sulfadiazine
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Sulfadiazine contains NLT 98.0% and NMT 102.0% of sulfadiazine, calculated on the dried basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B.
Sample: 50 mg
Analysis: Carefully melt the Sample in a small test tube.
Acceptance criteria: A reddish brown color develops upon melting, and the fumes evolved during decomposition do not discolor moistened lead acetate test paper (distinction from sulfathiazole).
C.
Sample: 1 g
Analysis 1: Gently heat the Sample in a small test tube until a sublimate is formed. Collect a few milligrams of the sublimate with a glass rod, and mix in a test tube with 1 mL of a solution (1 in 20) of Resorcinol in alcohol. Add 1 mL of sulfuric acid, and mix by shaking.
Acceptance criteria 1: A deep red color appears at once.
Analysis 2: Cautiously dilute the mixture obtained in Analysis 1 with 25 mL of ice-cold water, and add an excess of 6 N ammonium hydroxide.
Acceptance criteria 2: A blue or reddish blue color is produced.
3 ASSAY
Procedure
Mobile phase: Acetonitrile, water, and glacial acetic acid (12:87:1)
Standard solution: 1 mg/mL of USP Sulfadiazine RS in 0.025 N sodium hydroxide
Sample solution: 1 mg/mL of Sulfadiazine in 0.025 N sodium hydroxide
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4-mm × 30-cm; packing L1
Flow rate: 2 mL/min
Injection volume: 10 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of sulfadiazine in the portion of Sulfadiazine taken:
Result = (rU/rS) × (CS /CU) × 100
rU = peak response of sulfadiazine from the Sample solution
rS = peak response of sulfadiazine from the Standard solution
CS = concentration of USP Sulfadiazine RS in the Standard solution (mg/mL)
CU = concentration of Sulfadiazine in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the dried basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.1%
Delete the following:
Selenium 〈291〉
Sample: 200 mg
Acceptance criteria: NMT 30 ppm (USP 1-Dec-2021)
Ordinary Impurities 〈466〉
Diluent: Toluene and dimethylformamide (2:1)
Standard solutions: 0.008, 0.041, 0.08, and 0.17 mg/mL of USP Sulfadiazine RS in Diluent
Sample solution: 8.3 mg/mL of Sulfadiazine in Diluent
Eluant: Chloroform, methanol, and ammonium hydroxide (30:12:1)
Visualization: 11
Acceptance criteria: Meets the requirements
5 SPECIFIC TESTS
Clarity and Color of Solution
Sample: 1 g
Analysis: Dissolve the Sample in a mixture of 20 mL of water and 5 mL of 1 N sodium hydroxide.
Acceptance criteria: The solution is clear and not more deeply colored than pale yellow.
Acidity
Sample: 2 g
Analysis: Digest the Sample with 100 mL of water at about 70° for 5 min. Cool at once to room temperature, and filter. To 25.0 mL of the filtrate add 2 drops of phenolphthalein TS and titrate with 0.10 N sodium hydroxide.
Acceptance criteria: NMT 0.20 mL is required to produce a pink color.
Loss on Drying 〈731〉
Analysis: Dry at 105° for 2 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed, light-resistant containers.
USP Reference Standards 〈11〉
USP Sulfadiazine RS


