Sucrose Diacetate Hexaisobutyrate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C40H62O19 846.92
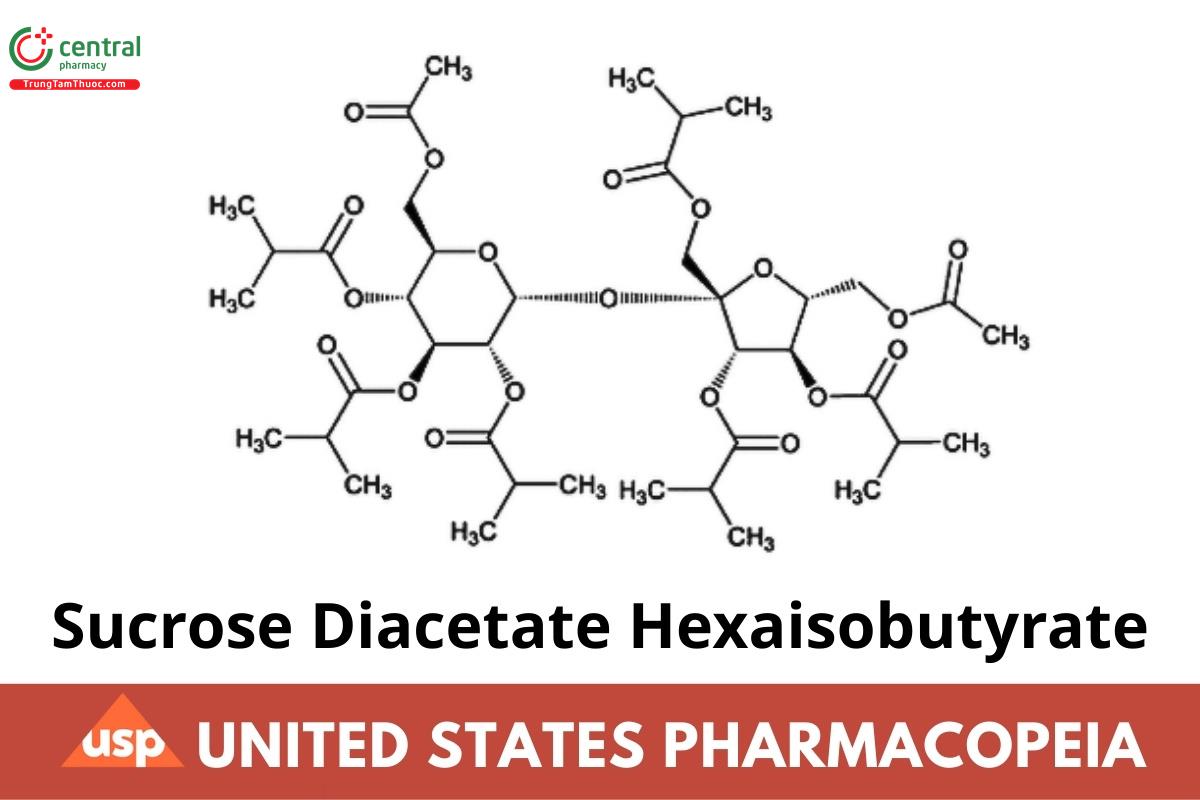
α-d-Glucopyranoside, 6-O-acetyl-1,3,4-tris-O-(2-methyl-1-oxopropyl)-β-d-fructofuranosyl, 6-acetate 2,3,4-tris(2-methylpropanoate);
6-O-Acetyl-1,3,4-tri-O-isobutyryl-β-d-fructofuranosyl 6-O-acetyl-2,3,4-tri-O-isobutyryl-α-d-glucopyranoside CAS RN®: 126-13-6.
1 DEFINITION
Sucrose Diacetate Hexaisobutyrate consists of a mixture of sucrose esters of acetic and isobutyric acid, with sucrose diacetate hexaisobutyrate being the predominant sucrose ester. It is produced through the controlled esterification of sucrose with acetic anhydride and isobutyric anhydride.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197F
B. It meets the requirements in the test for Saponification Value under Specific Tests.
3 ASSAY
3.1 Procedure
Analysis
Determine the saponification value, Sᵥ, as directed in the test for Saponification Value under Specific Tests.
Calculate the percentage of sucrose diacetate hexaisobutyrate (C40H62O19) in the portion of Sucrose Diacetate Hexaisobutyrate taken:
Result = [(SV × Mr1)/(Mr2 × N)] × F × 100
SV = saponification value
Mr1 = molecular weight of sucrose diacetate hexaisobutyrate, 846.92 g/mol
Mr2 = molecular weight of potassium hydroxide, 56.11 g/mol
N = ester group number per sucrose diacetate hexaisobutyrate molecule, 8
F = conversion factor, 10⁻³ g/mg
Acceptance criteria: 98.8%–101.9%
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.05%
5 SPECIFIC TESTS
5.1 Fats and Fixed Oils 〈401〉, Procedures, Acid Value
Analysis: Add 100 mL of acetone and 0.5 mL of bromothymol blue TS to a 500-mL Erlenmeyer flask. While stirring, titrate the solvent to a bromothymol blue endpoint, with 0.05 N sodium hydroxide. Place the flask with the blanked solvent on a balance and tare. Add between 8–12 g of sample into the flask. [Note—The sample needs to be heated first to become a pourable liquid.1] The solution will turn yellow if acid is present. If 12 g of added sample does not turn the solution yellow, additional sample may be added up to 100 g by increasing the weight incrementally. If the blue color of the solution is not changed by 100 g of sample after mixing, report the acid value to less than 0.006 (reporting limit). Swirl the contents of the flask to mix. Heat if necessary to dissolve the sample. If heating is required, allow the solution to cool to room temperature before titration. While stirring, titrate the sample with 0.05 N sodium hydroxide to the same blue endpoint as the blank.
Acceptance criteria: NMT 0.2
5.2 Fats and Fixed Oils 〈401〉, Procedures, Saponification Value
Analysis: Weigh accurately about 1.5 g of Sucrose Diacetate Hexaisobutyrate directly into a tared 500-mL Erlenmeyer flask. Accurately dispense or pipet 50.0 mL of 0.5 N alcoholic potassium hydroxide VS into the 500-mL Erlenmeyer flask containing the sample and also into a second 500-mL Erlenmeyer flask to be used for the blank. Add a couple of boiling chips to each flask. Place each flask on a hot plate and connect the flasks to the reflux condensers, which are cooled with water. Reflux the solutions for approximately 1 h. Allow the flasks to cool, then wash down each condenser with approximately 50 mL of water. After the condensers drain, remove the flasks from the condensers. Add approximately 0.2 mL or 10 drops of phenolphthalein TS to each flask. Titrate the blank and sample solutions with 0.5 N hydrochloric acid VS until the pink color disappears.
Acceptance criteria: 524–540
5.3 Water Determination 〈921〉, Method I: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed containers.
USP Reference Standards 〈11〉
USP Sucrose Diacetate Hexaisobutyrate RS (NF 1-Aug-2020)
1 Heating using a microwave oven for 1 min has been proven to be sufficient, and other equivalent conditions can also be used.


