Succinylcholine Chloride
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
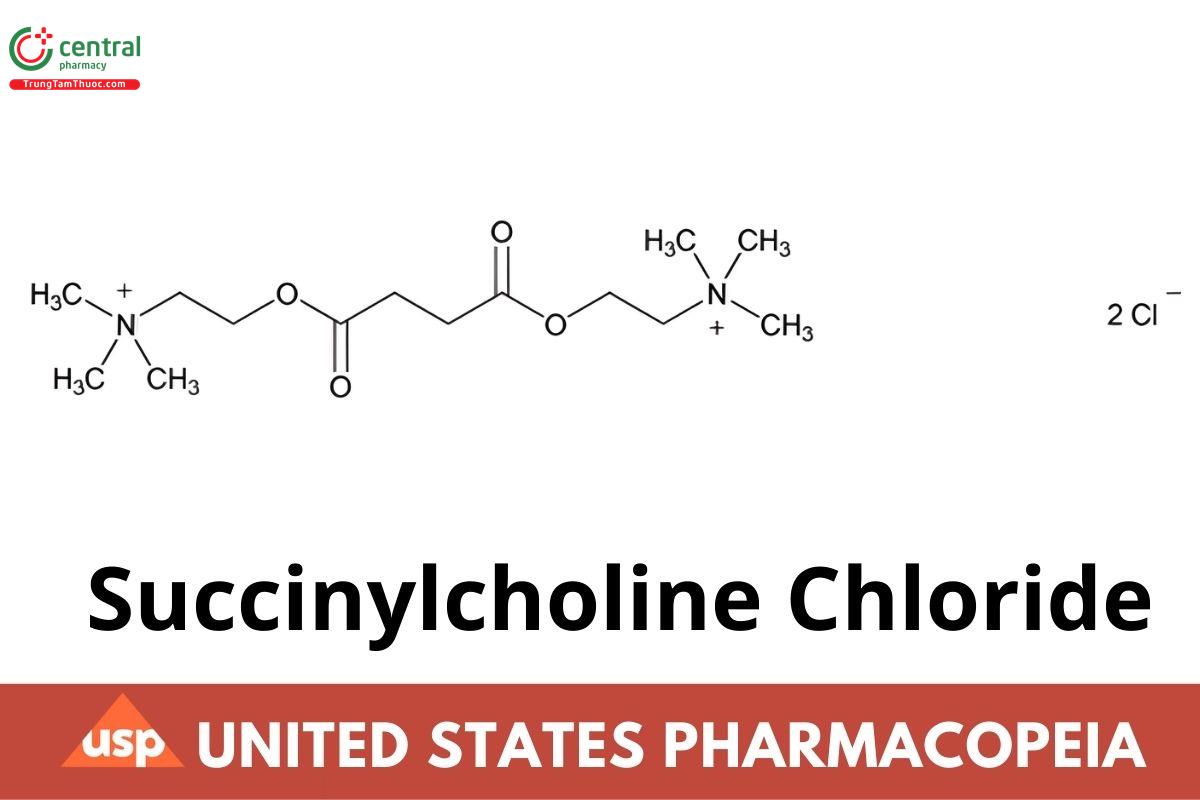
C14H30Cl2N2O4 (anhydrous) 361.31
C14H30Cl2N2O4 · 2H2O 397.34
Ethanaminium, 2,2'-[(1,4-dioxo-1,4-butanediyl)bis(oxy)]bis[N,N,N-trimethyl]-, dichloride;
Choline chloride succinate (2:1) CAS RN®: 71-27-2; UNII: I9L0DDD30I.
Dihydrate CAS RN®: 6101-15-1; UNII: 8L0S1G435E.
1 DEFINITION
Succinylcholine Chloride contains NLT 96.0% and NMT 102.0% of succinylcholine chloride (C14H30Cl2N2O4 ), calculated on the anhydrous basis.
[Caution—Succinylcholine chloride is a neuromuscular blocking agent. Great care should be taken when handling to avoid inhalation of dust or contact with skin.]
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
C. Identification Tests—General 〈191〉, Chemical Identification Tests, Chloride: Meets the requirements
3 ASSAY
Procedure
Mobile phase: Prepare a 1-in-10 solution of 1 N aqueous tetramethylammonium chloride in methanol. Adjust with hydrochloric acid to a pH of about 3.0.
Standard solution: 8.8 mg/mL of USP Succinylcholine Chloride RS prepared as follows. Transfer a suitable amount of USP Succinylcholine
Chloride RS to a suitable volumetric flask and dissolve in 40% of the total volume of water. Dilute with Mobile phase to volume while mixing.
Sample solution: 8.8 mg/mL of Succinylcholine Chloride prepared as follows. Transfer a suitable amount of Succinylcholine Chloride to a suitable volumetric flask and dissolve in 40% of the total volume of water. Dilute with Mobile phase to volume while mixing.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 214 nm
Column: 4-mm × 25-cm; 10-μm packing L3
Flow rate: 0.75 mL/min
Injection volume: 10 μL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.5
Relative standard deviation: NMT 1.5%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of succinylcholine chloride (C14H30Cl2N2O4) in the portion of Succinylcholine Chloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of succinylcholine chloride from the Sample solution
rS = peak response of succinylcholine chloride from the Standard solution
CS = concentration of USP Succinylcholine Chloride RS in the Standard solution (mg/mL)
CU = concentration of Succinylcholine Chloride in the Sample solution (mg/mL)
Acceptance criteria: 96.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.2%
Organic Impurities
Buffer: 3.85 g/L of anhydrous sodium 1-pentanesulfonate, 2.9 g/L of sodium chloride, and 1% (v/v) 1 N sulfuric acid in water
Mobile phase: Acetonitrile and Buffer (5:95)
System suitability solution: 0.5 mg/mL each of USP Citric Acid RS and USP Succinic Acid RS in Mobile phase
Standard solution: 0.05 mg/mL of USP Succinylmonocholine Chloride RS in Mobile phase
Sample solution: 10 mg/mL of Succinylcholine Chloride in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 214 nm
Column: 4.6-mm × 25-cm; 5-μm packing L1
Autosampler temperature: 4°
Flow rate: 1 mL/min
Injection volume: 50 μL
System suitability
Samples: System suitability solution and Standard solution
Suitability requirements
Resolution: NLT 2.9 between the citric acid and succinic acid peaks, System suitability solution
Relative standard deviation: NMT 3.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Succinylcholine Chloride taken:
Result = (rU/rS ) × (CS /CU ) × (1/F) × 100
rU = peak area of each impurity from the Sample solution
rS = peak area of succinylmonocholine chloride from the Standard solution
CS = concentration of USP Succinylmonocholine Chloride RS in the Standard solution (mg/mL)
CU = concentration of Succinylcholine Chloride in the Sample solution (mg/mL)
F = relative response factor (see Table 1)
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Edetate disodiuma | 0.18 | – | – |
| Succinic acid | 0.22 | 1.6 | 0.1 |
| Unidentified impurity 1b | 0.32 | 1.0 | 0.4 |
| Unidentified impurity 2b | 0.32 | 1.0 | |
| Succinylmonocholine | 0.49 | 1.0 | 0.4 |
| Succinylcholine | 1.0 | – | – |
| Any unspecified impurity | – | 1.0 | 0.2 |
| Total impuritiesb,c | – | – | 1.5 |
a Included for identifucation purposes only. Begin integration after this peak, if present.
b May occur as a doublet. Acceptance criteria is for the sum of both peaks.
c Total impurities include the sum of the results in the tests for Organic Impurities and Limit of Choline.
Limit of Choline
Solution A: 0.62 g/L of methanesulfonic acid
Solution B: 4.8 g/L of methanesulfonic acid
Mobile phase: See Table 2. Pre-equilibrate the instrument for NLT 3 min before each injection. [Note—Alternatively, the Mobile phase can be
generated electrolytically using an automatic eluant generator.]
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 14 | 100 | 0 |
| 15 | 0 | 100 |
| 33 | 0 | 100 |
| 34 | 100 | 0 |
| 40 | 100 | 0 |
System suitability solution: 10 μg/mL of USP Choline Chloride RS and 5 μg/mL of USP Potassium Chloride RS
Standard solution: 8 μg/mL of USP Choline Chloride RS
Sample solution: 2 mg/mL of Succinylcholine Chloride. Store at 4° immediately following preparation.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: Conductivity with suppression
Cell temperature: 35°
Columns
Guard: 2-mm × 5-cm; packing L98
Analytical: 2-mm × 25-cm; packing L97
Temperatures
Autosampler: 4°
Column: 35°
Flow rate: 0.25 mL/min
Injection volume: 5 μL
System suitability
Sample: System suitability solution
[Note—The relative retention times for potassium and choline are 0.6 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 5.0 between the choline and potassium peaks
Relative standard deviation: NMT 3.0% for choline
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of choline in the portion of Succinylcholine Chloride taken:
Result = (rU/rS) × (CS/CU) × (Mr1 /Mr2 ) × 100
rU = peak response of choline from the Sample solution
rS = peak response of choline from the Standard solution
CS = concentration of USP Choline Chloride RS in the Standard solution (mg/mL)
CU = concentration of Succinylcholine Chloride in the Sample solution (mg/mL)
Mr1 = molecular weight of choline, 104.17
Mr2 = molecular weight of choline chloride, 139.62
Acceptance criteria: NMT 0.3%
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I: NMT 10.0%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store at controlled room temperature.
Change to read:
USP Reference Standards 〈11〉
USP Choline Chloride RS
USP Citric Acid RS
USP Potassium Chloride RS
USP Succinic Acid RS
USP Succinylcholine Chloride RS
USP Succinylmonocholine Chloride RS
Ethanaminium, 2-(3- (ERR 1-Jan-2022) carboxy-1-oxopropoxy)-N,N,N-trimethyl-, chloride;
Also known as 2-[(3-Carboxypropanoyl)oxy]-N,N,N-trimethylethan-1-aminium chloride. (ERR 1-Jan-2022)
C9H18ClNO4 239.70


