Stearic Acid
If you find any inaccurate information, please let us know by providing your feedback here

This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
Portions of this monograph that are national USP text, and are not part of the harmonized text, are marked with symbols (⧫⧫) to specify this fact.
Octadecanoic acid;
Stearic acid
CAS RN®: 57-11-4.
1 DEFINITION
36 Mixture consisting of stearic (octadecanoic) acid (C18H36O2; Mr, 284.5) and palmitic (hexadecanoic) acid (C16H32O2; Mr, 256.4) obtained from fats or oils of vegetable or animal origin.
Content:
| Stearic acid 50 | Stearic acid: 40.0%–60.0% Sum of the contents of stearic acid and palmitic acids: NLT 90.0% |
| Stearic acid 70 | Stearic acid: 60.0%–80.0% Sum of the contents of stearic and palmitic acids: NLT 90.0% |
| Stearic acid 95 | Stearic acid: NLT 90.0% Sum of the contents of stearic acid and palmitic acids: NLT96.0% |
⧫[NOTE-Stearic Acid labeled solely for external use is exempt from the requirement that it be prepared from edible sources.]⧫
2 IDENTIFICATION
A. It meets the requirements of the test for Freezing Point.
B. ACID VALUE
Light petroleum: Use a sample that has the following properties: a clear, colorless liquid without fluorescence; practically insoluble in water; miscible with alcohol; density at 20° about 0.720; distillation range 100°-120°; water content NMT 0.03%.1
Sample solution: Dissolve 0.5 g of Stearic Acid in 50 mL of a mixture of equal volumes of alcohol and Light petroleum previously neutralized with 0.1 N potassium hydroxide or 0.1 N sodium hydroxide, using 0.5 mL of phenolphthalein TS as indicator. If necessary, heat to about 90° to dissolve the substance to be examined.
Analysis: Titrate the Sample solution with 0.1 N potassium hydroxide or 0.1 N sodium hydroxide until the pink color persists for at least 15 s. When heating has been applied to aid dissolution, maintain the temperature at about 90° during the titration.
Calculate the acid value (1) of the portion of Stearic Acid taken:
Result = (n/m) x N x Mr
n = amount of titrant used (mL)
m = amount of Stearic Acid taken to prepare the Sample solution (g)
N = normality of the potassium hydroxide solution
Mr = molecular weight of potassium hydroxide, 56.11
Acceptance criteria: 194-212
C. The retention times of the major peaks of the Sample solution correspond to those of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 PROCEDURE
Boron trifluoride-methanol solution: 140 g/L of boron trifluoride in methanol
Sample solution: Dissolve 100 mg of Stearic Acid in a small conical flask fitted with a suitable reflux attachment with 5 mL of Boron trifluoride-methanol solution. Boil under reflux for 10 min. Add 4.0 mL of heptane through the condenser, and boil again under reflux for 10 min. Allow to cool. Add 20 mL of a saturated solution of sodium chloride. Shake, and allow the layers to separate. Remove about 2 mL of the organic layer, and dry it over 0.2 g of anhydrous sodium sulfate. Dilute 1.0 mL of this solution with heptane to 10.0 mL.
Standard solution: Prepare as directed in the Sample solution using 50 mg of USP Stearic Acid RS and 50 mg of USP Palmitic Acid RS.
3.2 Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm x 30-m fused silica coated with a 0.5-µm layer of stationary phase G16
Temperatures
Injection port: 220°
Detector: 260°
Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 70 | — | 70 | 2 |
| 70 | 5 | 240 | 5 |
Carrier gas: Helium, passed through a bed of molecular sieve for drying, if necessary
Flow rate: 2.4 mL/min
Injection volume: 1 µL
3.3 System suitability
Sample: Standard solution
Suitability requirements
stearate Resolution: NLT 5.0 between the methyl palmitate and methyl stearate peaks determined on 6 injections
Relative standard deviation: NMT 3.0% for the methyl stearate and methyl palmitate peaks, from 6 replicate injections; NMT 1.0% for the ratio of the peak areas of methyl palmitate to the peak areas of methyl stearate, from 6 replicate injections
3.4 Analysis
Sample: Sample solution
Calculate the percentage of stearic acid (C18H36O2) in the portion of Stearic Acid taken:
Result = (AS/AT) x 100
AS = peak area due to methyl stearate
AT = sum of the peak areas of all the fatty acid esters
Calculate the percentage of palmitic acid (C16H32O2) in the portion of Palmitic Acid taken:
Result = (AP/AT) x 100
AP = peak area due to methyl palmitate
AT = sum of the peak areas of all the fatty acid esters
3.5 Acceptance criteria
For stearic acid 50: 40.0%-60.0% of stearic (octadecanoic) acid (C18H36O2), and the sum of the stearic acid and palmitic acid is NLT 90.0%.
For stearic acid 70: 60.0%-80.0% of stearic (octadecanoic) acid (C18H36O2), and the sum of the stearic acid and palmitic acid is NLT 90.0%.
For stearic acid 95: NLT 90.0% of stearic (octadecanoic) acid (C18H36O2), and the sum of the stearic acid and palmitic acid is NLT 96.0%.
4 IMPURITIES
⧫RESIDUE ON IGNITION (281): NMT 4 mg, determined on a 4-g portion (0.1%).⧫
5 SPECIFIC TESTS
5.1 FATS AND FIXED OILS (401), Procedures, lodine Value, Method I
Sample: 1 g
Analysis: Proceed as directed except use 15 mL of chloroform.
Acceptance criteria: See Table 2.
Table 2
| Type | Iodine Value |
| Stearic acid 50 | NMT 4.0 |
| Stearic acid 70 | NMT 4.0 |
| Stearic acid 95 | NMT 1.5 |
5.2 ⧫COLOR OF SOLUTION
Standard stock solution Y (yellow): 2.4 mL of ferric chloride CS, 0.6 mL of cobaltous chloride CS, and 7.0 mL of hydrochloric acid solution (10 g/L)
Standard stock solution BY (brownish-yellow): 2.4 mL of ferric chloride CS, 1.0 mL of cobaltous chloride CS, 0.4 mL of cupric sulfate CS, and 6.2 mL of hydrochloric acid solution (10 g/L)
Standard solution Y: 2.5 mL of Standard stock solution Y and 97.5 mL of hydrochloric acid solution (10 g/L)
Standard solution BY: 2.5 mL of Standard stock solution BY and 97.5 mL of hydrochloric acid solution (10 g/L)
Analysis: Heat Stearic Acid to 75°.
Acceptance criteria: The resulting liquid is not more intensely colored than Standard solution Y or Standard solution BY.⧫
5.3 ACIDITY
Sample: 5.0 g of Stearic Acid
Analysis: Melt the Sample, shake for 2 min with 10 mL of hot carbon dioxide-free water, cool slowly, and filter. To the filtrate add 0.05 mL of methyl orange TS.
Acceptance criteria: No red color develops.
Change to read:
5.4 FREEZING POINT
[NOTE-The following two procedures are equivalent and interchangeable. Only one procedure needs to be performed.]
5.4.1 Procedure 1 (NF 1-Aug-2023)
Apparatus: Consists of a test tube about 25 mm in diameter and 150 mm long placed inside a test tube about 40 mm in diameter and 160 mm long. The inner tube is closed by a stopper which carries a thermometer about 175 mm long and graduated in 0.2°, fixed so that the bulb is about 15 mm above the bottom of the tube. The stopper has a hole allowing the passage of the stem of a stirrer made from a glass rod or other suitable material formed at one end into a loop of about 18 mm overall diameter at right angles to the rod. The inner tube with its jacket is supported centrally in a 1-L beaker containing a suitable cooling liquid to within 20-mm of the top. A thermometer is supported in the cooling bath. In the inner tube, add sufficient quantity of the liquid or previously melted substance to be examined to cover the thermometer bulb, and determine the approximate freezing point by cooling rapidly.
Analysis: Place the inner tube in a bath about 5° above the approximate freezing point until all but the last traces of crystals are melted. Fill the beaker with water or a saturated solution of sodium chloride; at a temperature about 5° lower than the expected freezing point, insert the inner tube into the outer tube, ensuring that some seed crystals are present, and stir thoroughly until solidification takes place. Note the highest temperature observed during solidification.
5.4.2 Procedure 2
Alternatively, the freezing point is measured by the following procedure.
Apparatus: Use the apparatus illustrated in Figure 1. In the sample container (B), add sufficient quantity of the liquid or previously melted substance to be examined to the marked line (C). Adjust the immersion line (H) of the thermometer (F) to the same level of the meniscus of the sample and determine the approximate freezing point by cooling rapidly.
Analysis: Place the sample container (B) in a bath about 5° above the approximate freezing point until all but the last traces of crystals are melted. Fill the apparatus bath (D) with water or a saturated solution of sodium chloride at a temperature about 5° lower than the expected freezing point, then insert the sample container (B) into the cylinder (A), ensuring that some seed crystals are present, and stir thoroughly until solidification takes place. Note the highest temperature observed during solidification.
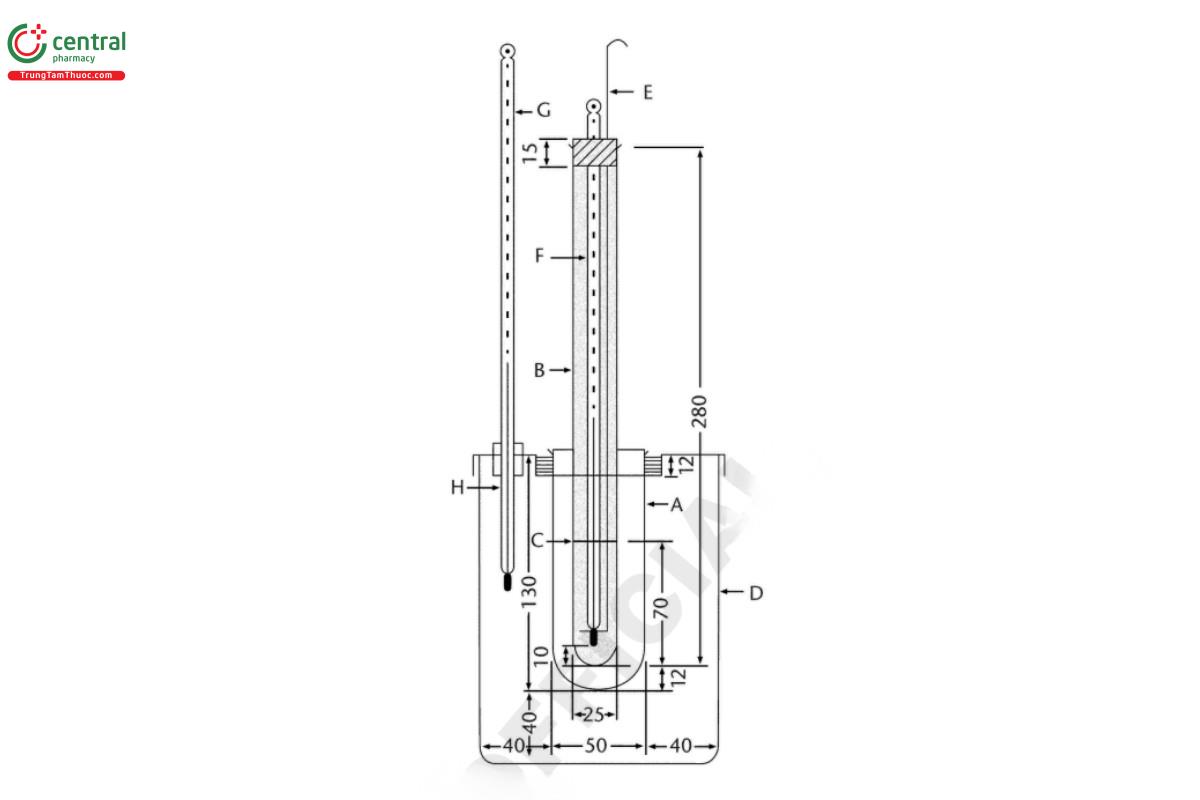
(NF 1-Aug-2023)
Acceptance criteria: See Table 3.
Table 3
| Type | Freezing Point (°) |
| Stearic acid 50 | 53–59 |
| Stearic acid 70 | 57–64 |
| Stearic acid 95 | 64–69 |
6 ADDITIONAL REQUIREMENTS
6.1 ⧫PACKAGING AND STORAGE
Preserve in well-closed containers.⧫
6.2 LABELING
⧫If it is for external use only, the labeling so indicates.⧫ The label states the type of stearic acid (50, 70, or 95).
6.3 USP REFERENCE STANDARDS (11)
USP Palmitic Acid RS
USP Stearic Acid RS
1 Petroleum ether; boiling range 100°-140°; CAS 64742-49-0 from Fisher Scientific; catalog number AC23302-0025 is suitable.


