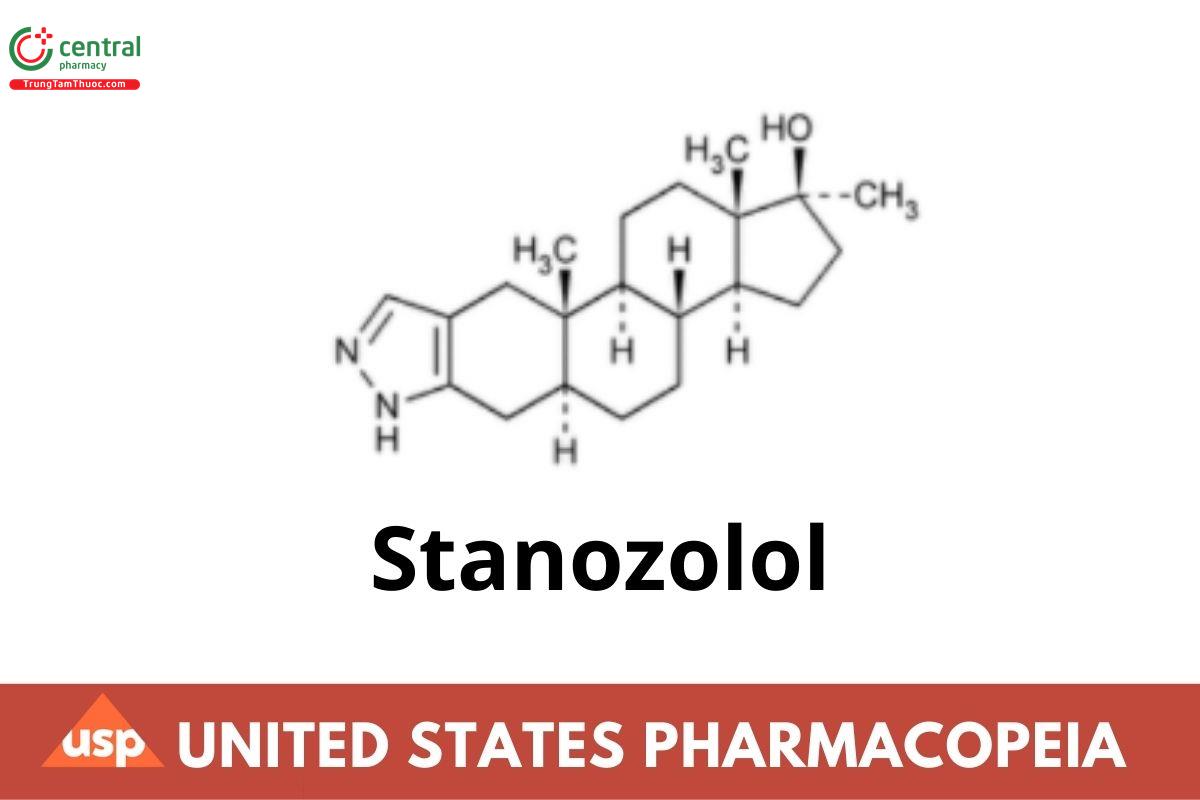
Stanozolol
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 Packaging and storage
Preserve in tight, light-resistant containers.
USP Reference standards 〈11〉—
USP Stanozolol RS
2 Identification
Change to read:
A: Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020) .
Change to read:
B: Spectroscopic Identification Tests 〈197〉, Ultraviolet-Visible Spectroscopy: 197U (CN 1-May-2020) —
Solution: 50 μg per mL.
Medium: alcohol.
Absorptivities at 224 nm, calculated on the dried basis, do not differ by more than 3.0%.
Specific rotation 〈781S〉: between +34° and +40°.
Test solution: 10 mg per mL, in chloroform.
Loss on drying 〈731〉—Dry it at a pressure not exceeding 5 mm of mercury at 100° to constant weight: it loses not more than 1.0% of its weight.
3 Chromatographic purity
Standard dilutions—Dissolve an accurately weighed quantity of USP Stanozolol RS in a mixture of chloroform and methanol (9:1) to obtain a solution having a known concentration of about 20 mg per mL. Dilute this solution with the same medium to obtain Standard dilutions having known concentrations of about 50, 100, 200, and 400 μg per mL, respectively.
Procedure—Score a 20- × 20-cm thin-layer chromatographic plate coated with a 0.25-mm layer of chromatographic silica gel mixture (binder-free) into channels 10 mm wide. Apply 10-μL portions, in two 5-μL increments, of a test solution prepared by dissolving Stanozolol in a mixture of chloroform and methanol (9:1) to obtain a solution containing about 20 mg per mL, and of each of the four Standard dilutions in the center of the channels at points about 2.5 cm from one edge of the plate. Develop the plate in a suitable chamber, lined with filter paper and previously equilibrated with 200 mL of a mixture of chloroform and methanol (188:12), for 15 minutes, taking care to ensure that the filter paper has been wetted completely with the solvent mixture. Allow the plate to develop until the solvent front has moved about 15 cm above the line of application. Remove the plate, and allow the solvent to evaporate completely. Spray it with 20% sulfuric acid, and heat in an oven at 100° for 15 minutes. Examine the plate under long-wavelength UV light: the channel for the test solution exhibits its principal spot at the same R value as the spots for the Standard dilutions. Estimate the concentration of any spots in the channel for the test solution, other than the principal spot, by comparison with the spots from the Standard dilutions. The spots from the 50-, 100-, 200-, and 400-μg-per-mL dilutions correspond to 0.25%, 0.5%, 1.0%, and 2.0% of chromatographic impurities, respectively, and the sum of the chromatographic impurities in the test solution is not greater than 2.0%.
4 Assay
Dissolve about 700 mg of Stanozolol, accurately weighed, in 50 mL of glacial acetic acid, add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 32.85 mg of C21H32N2O.


