Squalane
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
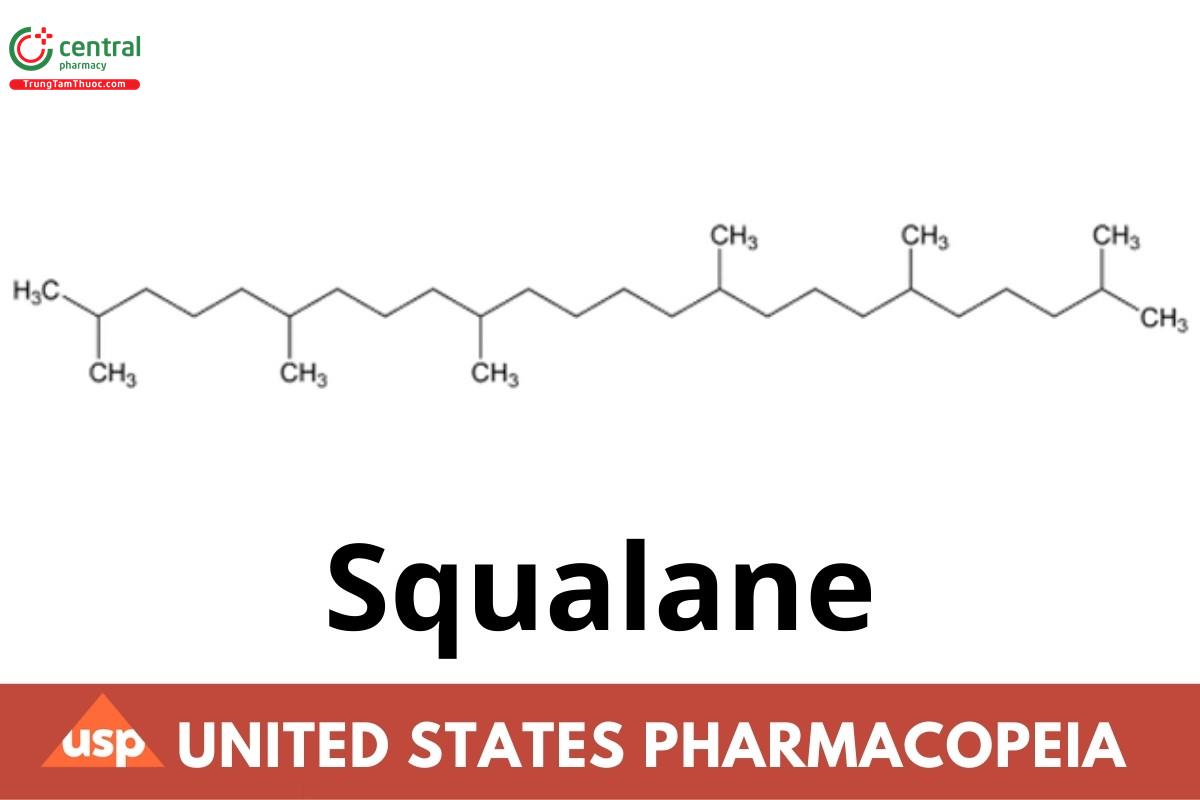
Squalane is a saturated hydrocarbon that contains NLT 97.0% and NMT 102.0% of 2,6,10,15,19,23-hexamethyltetracosane (C H ). It is obtained either by hydrogenation of squalene, an aliphatic triterpene derived from certain fish oils, particularly shark liver oil, or from β- farnesene, a fermentation product of plant sugars.
2 IDENTIFICATION
Change to read:
A. SPECTROSCOPIC IDENTIFICATION TESTS 〈197〉 , Infrared Spectroscopy: 197F (CN 1-M -2020)
Sample: Undried specimen
Acceptance criteria: Meets the requirements
B. CHROMATOGRAPHIC IDENTITY
Analysis: Proceed as directed in the Assay.
Acceptance criteria: The retention time of the major peak of the Sample solution, excluding the solvent peak, corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
PROCEDURE
Diluent: n-Heptane
System suitability solution: 4 mg/mL of USP Squalane RS and 4 µL/mL of methyl erucate in Diluent Standard solution: 4 mg/mL of USP Squalane RS in Diluent
Sample solution: 4 mg/mL of Squalane in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 30-m fused silica capillary; coated with a 1-µm film of phase G2
Temperatures
Injection port: 275°
Detector: 300° Column: See Table 1.
Table 1
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
| 60 | 5.9 | 290 | 11 |
Carrier gas: Helium Flow rate: 1.7 mL/min
Injection volume: 1 µL
Injection type: Split; split ratio, 12:1
Liner: General purpose split/splitless liner with deactivated wool
System suitability
Samples: System suitability solution and Standard solution
[NOTE—The relative retention times for methyl erucate and squalane are 0.9 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 5 between the peaks due to methyl erucate and squalane, System suitability solution
Relative standard deviation: NMT 2%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of squalane (C H ) in the portion of Squalane taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak area from the Sample solution
rS = peak area from the Standard solution
CS = concentration of USP Squalane RS in the Standard solution (mg/mL)
CU = concentration of Squalane in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–102.0%
4 IMPURITIES
RESIDUE ON IGNITION 〈281〉: NMT 0.5%
5 SPECIFIC TESTS
SPECIFIC GRAVITY 〈841〉: 0.807–0.810 at 20°
FATS AND FIXED OILS 〈401〉 , Procedures, Acid Value: NMT 0.2
FATS AND FIXED OILS, Iodine Value 〈401〉: NMT 4
FATS AND FIXED OILS, Saponification Value 〈401〉: NMT 2
REFRACTIVE INDEX 〈831〉: 1.4510–1.4525 at 20°
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.
LABELING: Label to indicate whether it is derived from animal or plant sources.
USP REFERENCE STANDARDS 〈11〉
USP Squalane RS


