Sodium Tartrate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
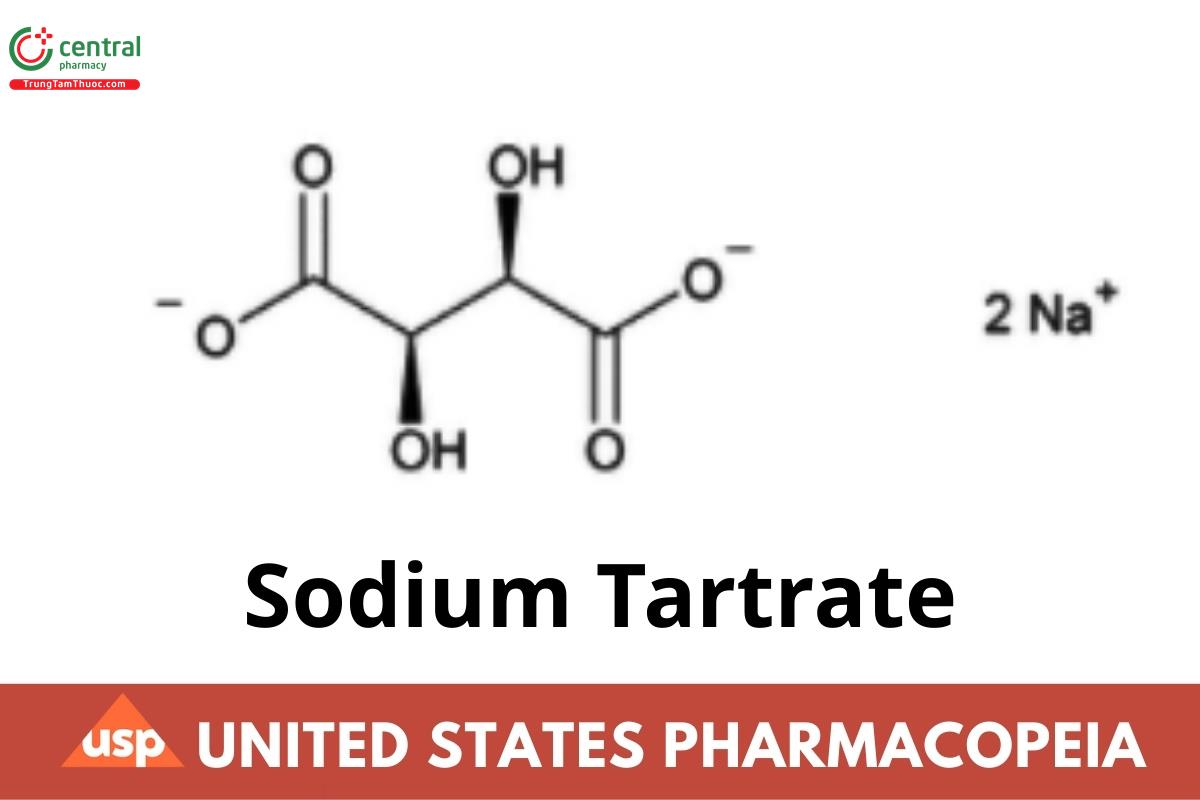
C4H4Na2O6 . 2H2O 230.08
Disodium L-tartrate;
Disodium (+)-2,3-dihydroxybutanedioic acid CAS RN®: 868-18-8.
1 DEFINITION
Sodium Tartrate contains NLT 99.0% and NMT 100.5% of sodium tartrate (C4H4Na2O6), calculated on the dried basis.
2 IDENTIFICATION
A. IDENTIFICATION TESTS-GENERAL, Sodium (191): Meets the requirements
B. IDENTIFICATION TESTS-GENERAL, Tartrate (191): Meets the requirements
3 ASSAY
3.1 PROCEDURE
Sample: 250 mg, previously dried at 150° for 3 h
Titrimetric system
(See Titrimetry (541).)
Mode: Direct titration
Titrant: 0.1 N Perchloric acid VS (in glacial acetic acid)
Endpoint detection: Potentiometric
Analysis: Dissolve the Sample in 150 mL of glacial acetic acid by stirring and heating to near the boiling point. Cool to room temperature. Titrate with 0.1 N perchloric acid VS (in glacial acetic acid) to a potentiometric endpoint. Perform a blank determination, and make any necessary adjustments. Each mL of 0.1 N perchloric acid is equivalent to 9.703 mg of sodium tartrate (C4H4Na2O6).
Acceptance criteria: 99.0%-100.5% on the dried basis
4 SPECIFIC TESTS
PH (791): 7-9 (1 in 10 solution)
LOSS ON DRYING (731): Dry a sample at 150° for 3 h: it loses 14.0%-17.0% of its weight.
5 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in a tight container. No storage requirements specified.


