Sodium Lactate Injection
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
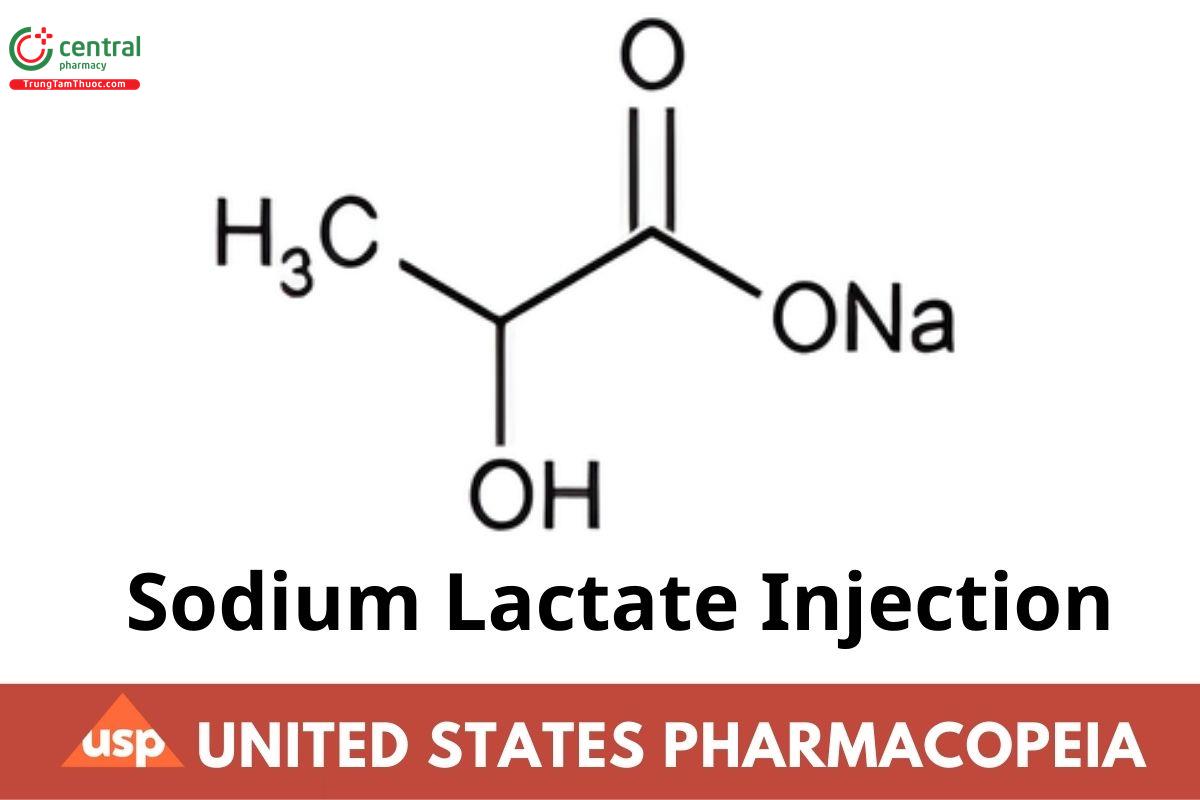
C3H5NaO3 112.06
Propanoic acid, 2-hydroxy-, monosodium salt.
Sodium lactate CAS RN®: 72-17-3.
» Sodium Lactate Injection is sterile Sodium Lactate Solution in Water for Injection, or a sterile solution of Lactic Acid in Water for Injection prepared with the aid of Sodium Hydroxide. It contains not less than 95.0 percent and not more than 110.0 percent of the labeled amount of C3H5NaO3 .
Packaging and storage—Preserve in single-dose glass or plastic containers. Glass containers are preferably of Type I or Type II glass.
Labeling—The label states the total osmolar concentration in mOsmol per L. Where the contents are less than 100 mL, or where the label states that the Injection is not for direct injection but is to be diluted before use, the label alternatively may state the total osmolar concentration in mOsmol per mL. The label includes also the warning: “Not for use in the treatment of lactic acidosis.”
Identification—Overlay 2 mL of Injection on 5 mL of a 1 in 100 solution of catechol in sulfuric acid: a deep red color is produced at the zone of contact.
Bacterial Endotoxins Test 〈85〉 —It contains not more than 2.0 USP Endotoxin Units per mEq.
pH 〈791〉: between 6.0 and 7.3, the Injection being diluted with water, if necessary, to approximately 0.16 M (20 mg per mL).
Particulate Matter in Injections 〈788〉: meets the requirements under small-volume injections.
Other requirements—It meets the requirements under Injections and Implanted Drug Products 〈1〉.
Assay—Pipet into a small beaker a volume of Injection, equivalent to about 300 mg of sodium lactate, and evaporate to dryness. Add to the residue 60 mL of a 1 in 5 mixture of acetic anhydride in glacial acetic acid, and stir until the residue is completely dissolved. Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 11.21 mg of C3H5NaO3.


