Sodium Formaldehyde Sulfoxylate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
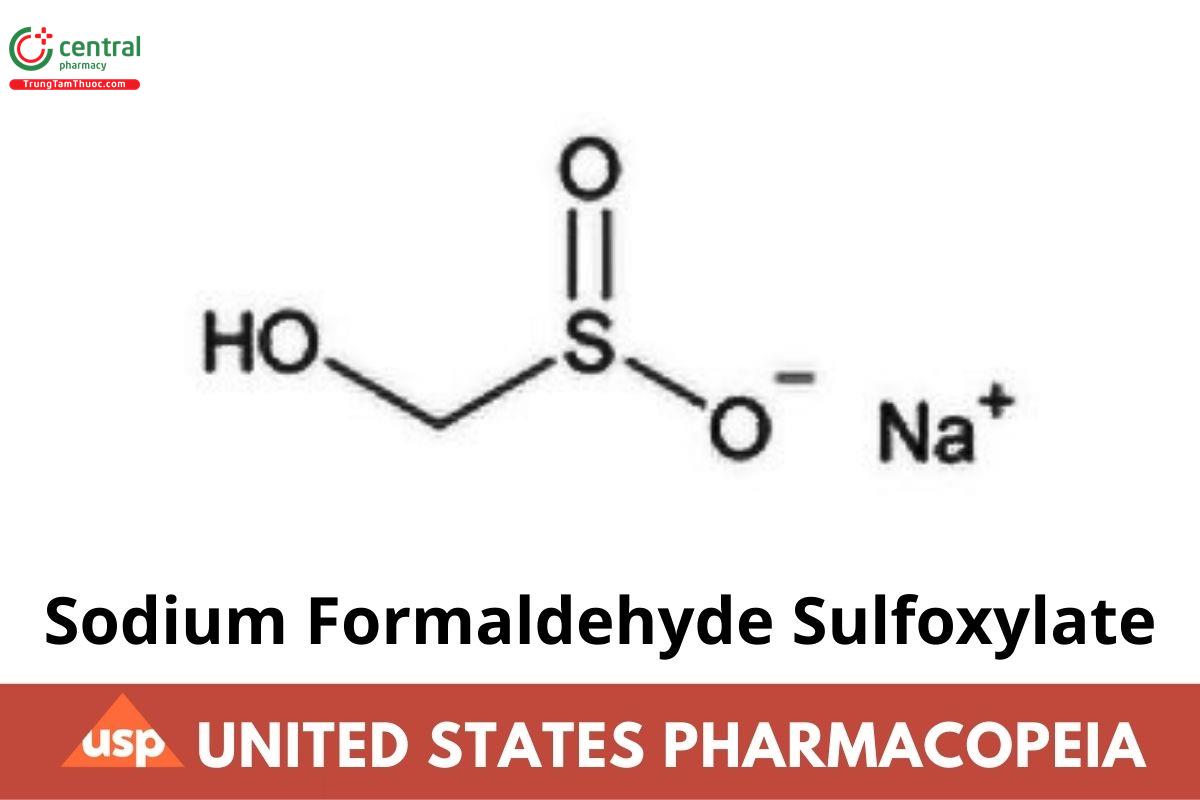
CH₃NaO₃S 118.09
CH₃NaO₃S · 2H₂O 154.11
Methanesulfinic acid, hydroxy-, monosodium salt;
Monosodium hydroxymethanesulfinate CAS RN®: 149-44-0.
Dihydrate CAS RN®: 6035-47-8.
1 DEFINITION
Sodium Formaldehyde Sulfoxylate contains an amount of sodium formaldehyde sulfoxylate (CH₃NaO₃S) equivalent to NLT 45.5% and NMT 54.5% of SO₂, calculated on the dried basis. It may contain a suitable stabilizer, such as sodium carbonate.
2 IDENTIFICATION
2.1 A.
Sample solution: Dissolve 4 g in 10 mL of water in a test tube.
Analysis: To the Sample solution add 1 mL of silver–ammonia–nitrate TS.
Acceptance criteria: Metallic silver is produced, either as a finely divided, gray precipitate or as a bright metallic mirror on the inner surface of the tube.
2.2 B.
Sample solution: Dissolve 40 mg of salicylic acid in 5 mL of sulfuric acid, and add 50 mg of Sodium Formaldehyde Sulfoxylate.
Analysis: Warm very gently.
Acceptance criteria: A permanent, deep red color appears.
3 ASSAY
3.1 Procedure
Sample: 1 g
Titrimetric system
- (See Titrimetry 〈541〉.)
- Mode: Direct titration
- Titrant: 0.1 N iodine VS. [Note-Prepare an adequate amount for both the Assay and the test for Sodium Sulfite.]
- Endpoint detection: Visual
Analysis: Transfer the Sample to a 50-mL volumetric flask, dissolve in 25 mL of water, and dilute with water to volume. Reserve a portion of this solution for the test for Sodium Sulfite. Transfer 4.0 mL of the remaining solution to a conical flask containing 100 mL of water. Titrate with Titrant, adding 3 mL of starch TS as the endpoint is approached. Each mL of 0.1 N iodine is equivalent to 1.602 mg of SO₂.
Acceptance criteria: 45.5%–54.5% of SO₂ on the dried basis
4 IMPURITIES
4.1 Sulfide
Analysis: Dissolve 6 g in 14 mL of water in a test tube, and wet a strip of lead acetate test paper with the clear solution.
Acceptance criteria: No discoloration is evident within 5 min.
4.2 Iron
Standard solution: Dissolve 43.2 mg of ferric ammonium sulfate in 10 mL of 2 N sulfuric acid, and add water to make 1000 mL, each mL representing 5 µg of Fe.
Sample solution: Transfer 1.0 g of Sodium Formaldehyde Sulfoxylate to a suitable crucible, and carefully ignite, initially at a low temperature until thoroughly charred, and finally, preferably in a muffle furnace, at 500°–600° until the carbon is all burned off. Cool, dissolve the residue in 2 mL of hydrochloric acid, and dilute with water to 50 mL.
Analysis: To 5.0 mL of the Standard solution and 50 mL of the Sample solution add 50 mg of ammonium persulfate and 5 mL of ammonium thiocyanate TS, and transfer each to a separate color comparison tube.
Acceptance criteria: 0.0025%; the color of the Sample is not deeper than that of the Standard solution.
4.3 Sodium Sulfite
Sample solution: 4.0 mL of the solution prepared for the Assay in a conical flask containing 100 mL of water
Titrimetric system
- (See Titrimetry 〈541〉.)
- Mode: Direct titration
- Titrant: 0.1 N iodine VS, prepared in the Assay
- Endpoint detection: Visual
Analysis: Add 2 mL of formaldehyde TS to the Sample solution, and titrate with the Titrant, adding 3 mL of starch TS as the endpoint is approached.
Calculate the percentage of sodium sulfite (Na₂SO₃) in the Sodium Formaldehyde Sulfoxylate taken:
Result = (V₂ − V₁) × (N/W) × (F × 1.25)
V₂ = volume of 0.1 N iodine VS consumed in the titration performed in the Assay (mL)
V₁ = volume of 0.1 N iodine VS consumed in this titration (mL)
N = actual normality of the Titrant (mEq/mL)
W = weight of the Sample in the Assay (g)
F = equivalency weight of sodium sulfite, 63.02 mg/mEq
Acceptance criteria: NMT 5.0% on the dried basis
5 SPECIFIC TESTS
5.1 pH 〈791〉
Sample solution: 20 mg/mL
Acceptance criteria: 9.5–10.5
5.2 Loss on Drying 〈731〉
Analysis: Dry at 105° for 3 h.
Acceptance criteria: NMT 27.0%
5.3 Alkalinity
Sample solution: 1.0 g of Sodium Formaldehyde Sulfoxylate in 50 mL of water
Analysis: To the Sample solution add phenolphthalein TS, and titrate with 0.10 N sulfuric acid.
Acceptance criteria: NMT 3.5 mL is required for neutralization.
5.4 Clarity and Color of Solution
Sample solution: 1 g of Sodium Formaldehyde Sulfoxylate in 20 mL of water
Analysis: Transfer 10 mL of the Sample solution to a 20- × 150-mm test tube. Compare with water in a similar test tube.
Acceptance criteria: The Sample solution and the water are equally clear and, when viewed transversely by transmitted light, exhibit no apparent difference in color.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed, light-resistant containers, and store at controlled room temperature.


