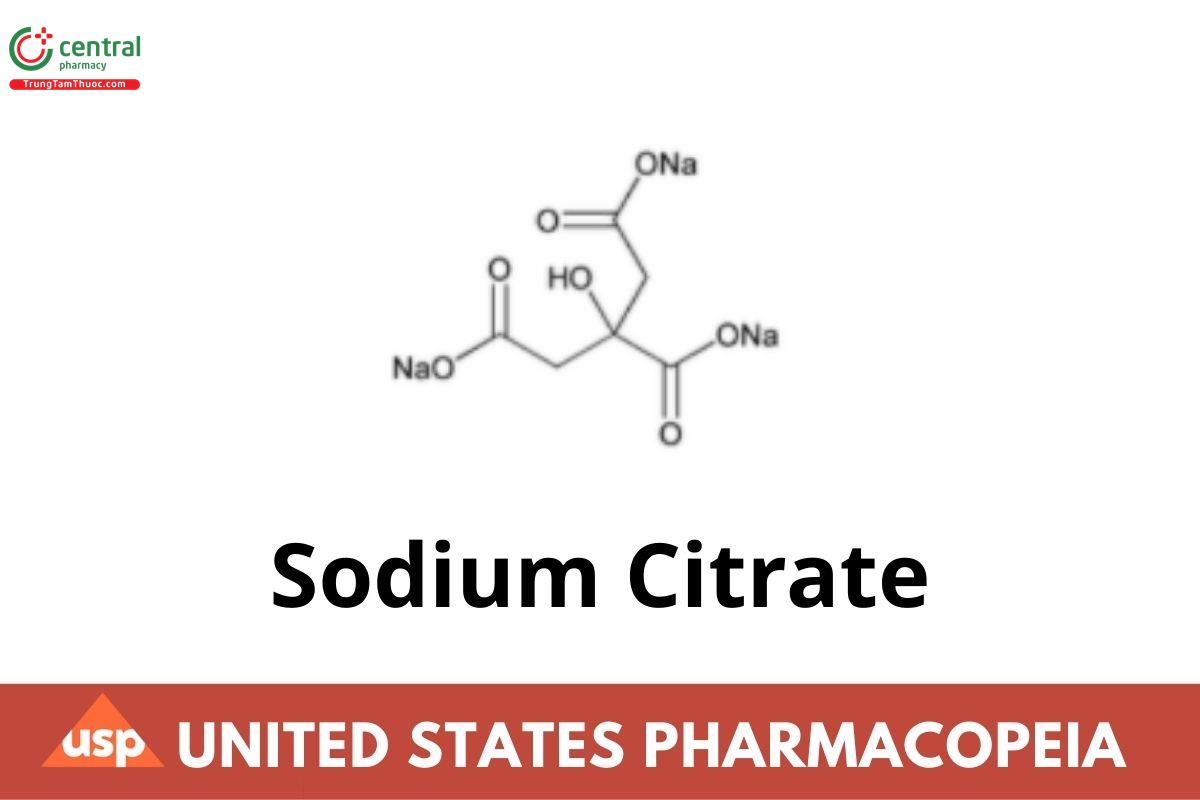
Sodium Citrate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
1 DEFINITION
Sodium Citrate is anhydrous or contains two molecules of water of hydration. It contains NLT 99.0% and NMT 100.5% of C6H5Na3O7, calculated on the anhydrous basis.
2 IDENTIFICATION
A. Identification Tests—General, Sodium〈191〉
Sample solution: 50 mg/mL
Acceptance criteria: Meets the requirements
B. Identification Tests—General, Citrate〈191〉
Sample solution: 50 mg/mL
Acceptance criteria: Meets the requirements
C. Upon ignition, it yields an alkaline residue that effervesces when treated with 3 N hydrochloric acid.
3 ASSAY
Procedure
Sample: Add 100 mL of glacial acetic acid to 350 mg of Sodium Citrate (previously dried at 180° for 18 h) in a 250-mL beaker. Stir until completely dissolved.
Analysis: Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction (see Titrimetry 〈541〉). Each mL of 0.1 N perchloric acid is equivalent to 8.602 mg of C6H5Na3O7.
Acceptance criteria: 99.0%–100.5% on the anhydrous basis
4 IMPURITIES
Tartrate
Analysis: To a solution of 1 g in 2 mL of water in a test tube add 1 mL of potassium acetate TS and 1 mL of 6 N acetic acid. Rub the wall of the tube with a glass rod.
Acceptance criteria: No crystalline precipitate is formed.
5 SPECIFIC TESTS
Alkalinity
Sample solution: 1.0 g in 20 mL of water
Acceptance criteria: The Sample solution is alkaline to litmus paper, but after the addition of 0.20 mL of 0.10 N sulfuric acid, no pink color is produced by 1 drop of phenolphthalein TS.
Water Determination, Method III〈921〉: Dry a sample at 180° for 18 h: the anhydrous form loses NMT 1.0% of its weight; the hydrous form loses 10.0%–13.0% of its weight.
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: Label it to indicate whether it is anhydrous or hydrous.


