Sodium Butyrate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
DOWNLOAD PDF HERE
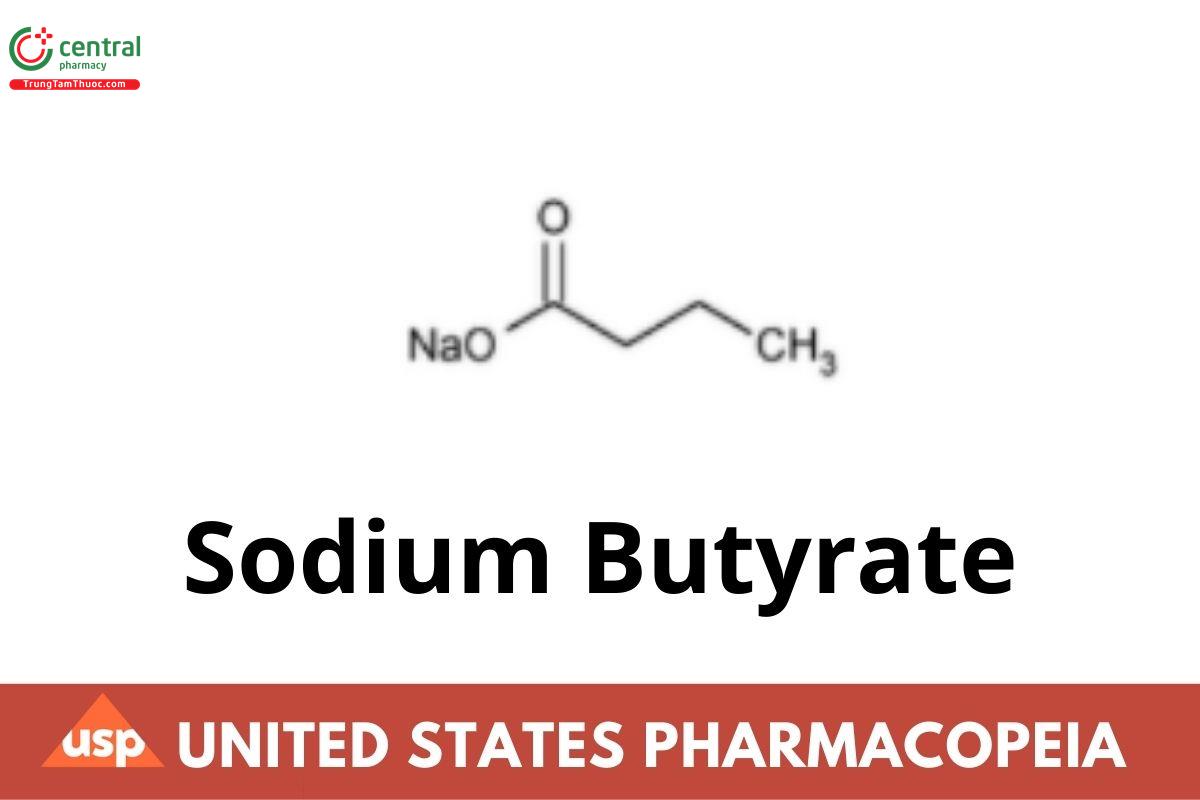
Sodium Butyrate contains not less than 98.0 percent and not more than 101.0 percent of C4H7NaO2, calculated on the anhydrous basis.
1 Packaging and storage
Preserve in tight containers, and store at controlled room temperature.
2 Labeling
Label it to indicate that it is intended for use in compounding dosage forms for rectal use only.
USP Reference standards 〈11〉—
USP Sodium Butyrate RS
3 Identification
Change to read:
A: Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K (CN 1-May-2020) , on the undried specimen.
B: A solution (1 in 10) meets the requirements of the tests for Sodium 〈191〉.
Alkalinity—Dissolve 2.0 g in 20 mL of water, and add 1 drop of phenolphthalein TS: if a pink color is produced, it is discharged by 0.50 mL of 0.10 N sulfuric acid.
Water Determination, Method I 〈921〉: not more than 1.0%.
4 Assay
Dissolve about 200 mg of Sodium Butyrate, accurately weighed, in 50 mL of glacial acetic acid. Add 1 drop of crystal violet TS, and fititrate with 0.1 N perchloric acid to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 11.01 mg of C4H7NaO2.


