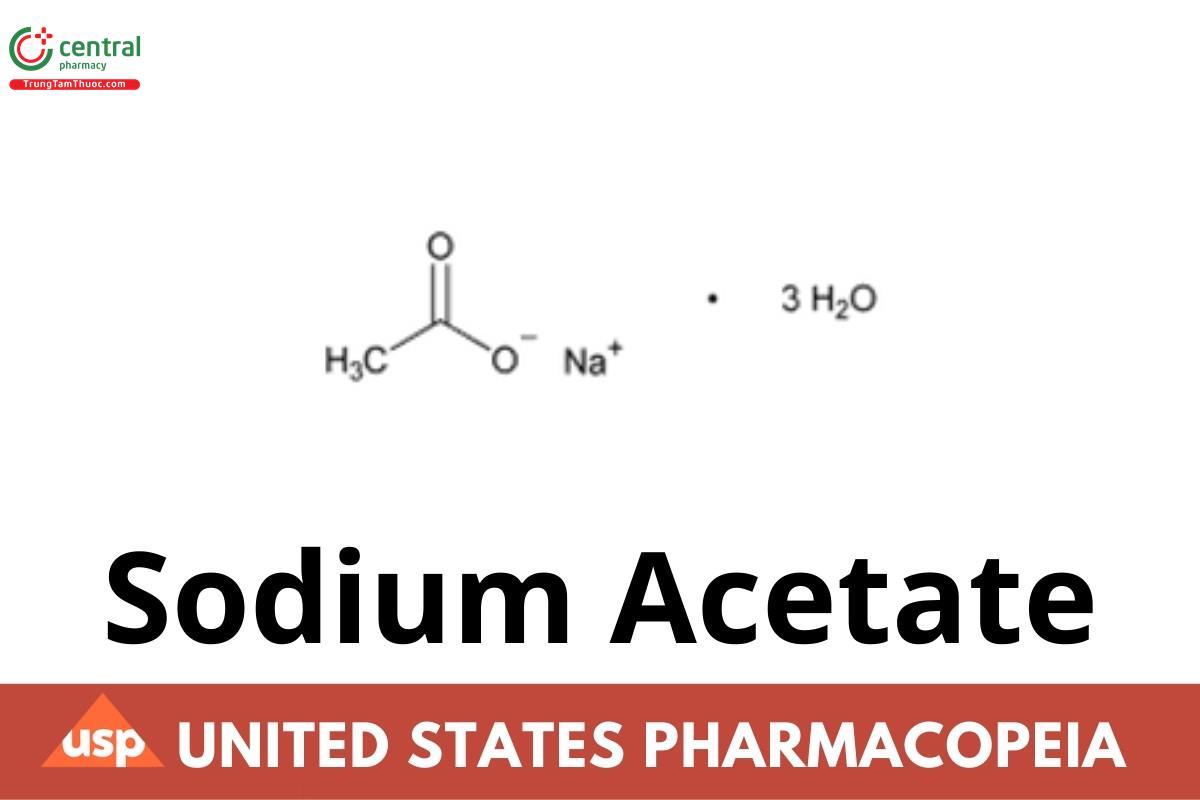
Sodium Acetate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Sodium Acetate contains three molecules of water of hydration, or is anhydrous. It contains NLT 99.0% and NMT 101.0% of sodium acetate (C2H3NaO2), calculated on the dried basis.
2 IDENTIFICATION
A. IDENTIFICATION TESTS—GENERAL 〈191〉 , Chemical Identification Tests, Sodium: Meets the requirements
B. IDENTIFICATION TESTS—GENERAL 〈191〉 , Acetate
Sample solution : 10 mg/mL of Sodium Acetate in water. Adjust with 0.1 N sodium hydroxide VS to a slightly alkaline pH. Acceptance criteria: Meets the requirements in test A
3 ASSAY
PROCEDURE
Sample solution: Equivalent to 200 mg of anhydrous Sodium Acetate in 50 mL of glacial acetic acid. Heat gently on a steam bath for about 5 min to dissolve. Cover the solution and stir while cooling.
Blank: 50 mL of glacial acetic acid
Titrimetric system (See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant: 0.1 N perchloric acid VS Endpoint detection: Potentiometric
Analysis: Titrate the Sample solution with Titrant. Perform a blank determination, and make any necessary correction. Calculate the percentage of sodium acetate (C H NaO ) in the portion of Sodium Acetate taken:
Result = [(VS − VB) × NA × F × 100]/W
VS = Titrant volume consumed by the Sample solution (mL)
VB = Titrant volume consumed by the Blank (mL)
NA = actual normality of the Titrant (mEq/mL)
F = equivalency factor, 82.03 mg/mEq
W = equivalent weight of anhydrous Sodium Acetate in the Sample solution (mg) Acceptance criteria: 99.0%–101.0% on the dried basis
4 IMPURITIES
4.1 CHLORIDE AND SULFATE 〈221〉 , Chloride
Sample: Equivalent to 1.0 g of anhydrous Sodium Acetate
Acceptance criteria: Shows no more chloride than corresponds to 0.50 mL of 0.020 N hydrochloric acid VS (350 ppm).
4.2 CHLORIDE AND SULFATE 〈221〉 , Sulfate
Sample: Equivalent to 10 g of anhydrous Sodium Acetate
Acceptance criteria: Shows no more sulfate than corresponds to 0.50 mL of 0.020 N sulfuric acid VS (50 ppm).
4.3 CALCIUM AND MAGNESIUM
Sample solution: Equivalent to 10 mg/mL of anhydrous Sodium Acetate
Analysis: To 20 mL of Sample solution, add 2 mL each of 6 N ammonium hydroxide, ammonium oxalate TS, and dibasic sodium phosphate TS.
Acceptance criteria: No turbidity is produced within 5 min.
4.4 POTASSIUM
Sample solution: Dissolve the equivalent of 3 g of anhydrous Sodium Acetate in 5 mL of water.
Analysis: To the Sample solution, add 1 N acetic acid dropwise until the solution is slightly acidic, and then add 5 drops of sodium cobaltinitrite TS.
Acceptance criteria: No precipitate is formed. Change to read:
4.5 ALUMINUM 〈206〉 , Procedure 1 (CN 1-JUN-2023) (where it is labeled as intended for use in hemodialysis)
Test preparation: Prepare as directed in the chapter, using a portion equivalent to 10 g of sodium acetate trihydrate. Acceptance criteria: 0.2 µg/g
5 SPECIFIC TESTS
5.1 PH 〈791〉
Sample solution: Equivalent to 30 mg/mL of anhydrous Sodium Acetate in carbon dioxide-free water Acceptance criteria: 7.5–9.2
5.2 LOSS ON DRYING 〈731〉
Analysis: Dry at 120° to constant weight. Acceptance criteria
Anhydrous: NMT 1.0%
Trihydrate: 38.0%–41.0%
5.3 INSOLUBLE MATTER
Sample: Equivalent to 20 g of anhydrous Sodium Acetate
Analysis: Dissolve the Sample in 150 mL of water, heat to boiling, and digest in a covered beaker on a steam bath for 1 h. Filter through a tared filtering crucible, wash thoroughly, and dry at 105°.
Acceptance criteria: The weight of the residue is NMT 10 mg (0.05%).
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in tight containers.
LABELING: Label it to indicate whether it is the trihydrate or is anhydrous. Where Sodium Acetate is intended for use in hemodialysis, it is so labeled.


