Shellac
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
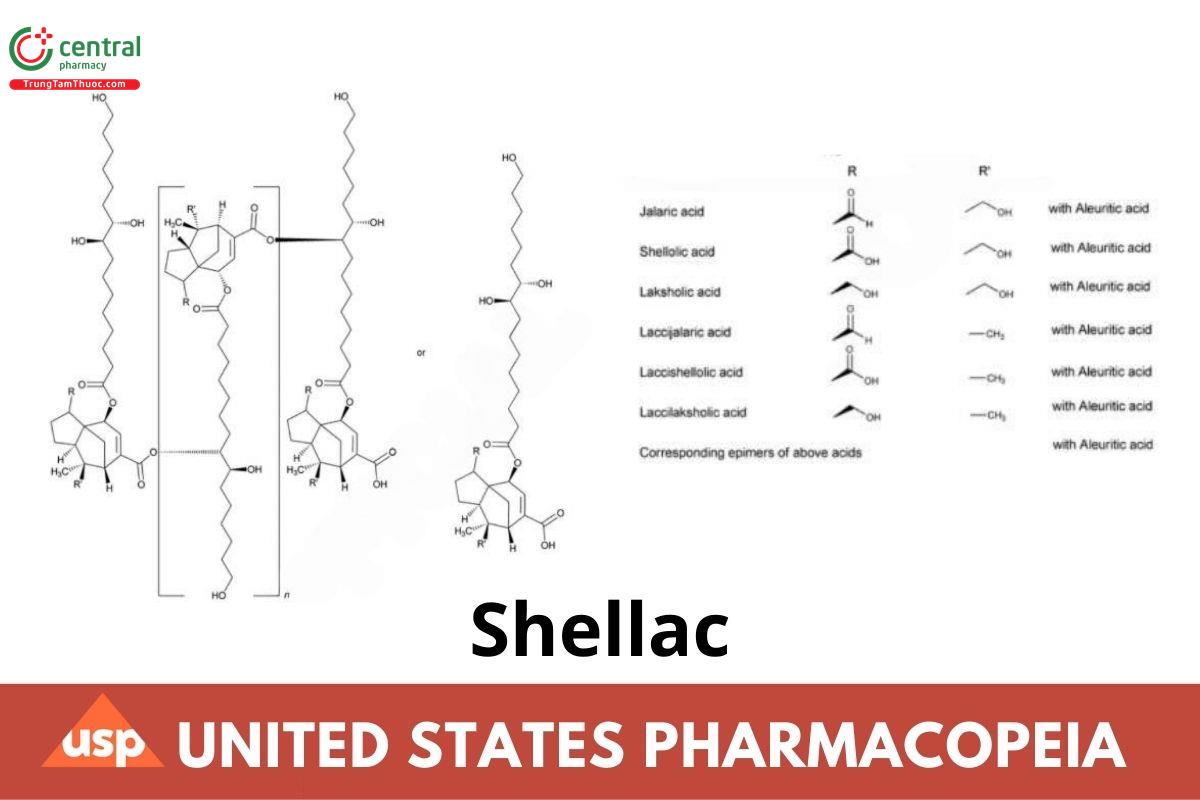
Shellac is obtained by the purification of lac, the resinuous secretion of the insect Kerria lacca (Kerr) Lindinger (Laccifer lacca Kerr) (Fam. Coccideae). Shellac is a polyester resin consisting of inter- and intra-esters of polyhydroxyl carboxylic acids formed from certain hydroxyl acids and sesquiterpenic acids, and also contains variable amounts of wax. There are four types of Shellac depending on the nature of the treatment of crude secretion (seedlac).
1. Orange Shellac is produced by a process of filtration in the molten state and/or by a process of solvent extraction. Orange Shellac retains most of its wax.
2. Refined (Dewaxed) Orange Shellac is produced by filtration of the wax in a solvent process. It may also be decolorized by activated carbon.
3. Regular Bleached (white) Shellac is prepared by dissolving the lac in an alkaline solution and bleaching the solution with sodium hypochlorite. It is precipitated by dilute acid and dried.
4. Refined Bleached Shellac is prepared by dissolving the lac in an alkaline solution and bleaching the solution with sodium hypochlorite. During the process, wax is removed by filtration. It is precipitated by dilute acid and dried.
Shellac conforms to the specifications in Table 1.
Table 1
| Acid Value (on dried basis) | Loss on Drying (%) | Wax (%) | |
| Orange Shellac | 68–76 | NMT 2.0 | NMT 5.5 |
| Refined Orange Shellac | 68–79 | NMT 2.0 | NMT 0.2 |
| Regular Bleached Shellac | 73–89 | NMT 6.0 | NMT 5.5 |
| Refined Bleached Shellac | 75–91 | NMT 6.0 | NMT 0.2 |
2 IDENTIFICATION
Change to read:
2.1 A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 1974 ог 197K. 197K (CN 1-MAY-2020)
Due to the degree of polymerization, the intensity of some absorption bands may vary.
Use USP Regular Bleached Shellac RS for the following two types:
- Orange Shellac
- Regular Bleached Shellac
Use USP Refined Bleached Shellac RS for the following two types:
- Refined Bleached Shellac
- Refined Orange Shellac
2.2 B. IDENTIFICATION OF ALEURITIC ACID AND SHELLOLIC ACID BY THIN-LAYER CHROMATOGRAPHY
Standard solution: 6 mg/mL of USP Aleuritic Acid RS in methanol, heating slightly if necessary.
Sample solution: Weigh and finely powder 500 mg of Shellac. Transfer 500 mg of the finely powdered Shellac to a test tube and heat with 4 mL of 85-mg/mL sodium hydroxide solution in a vigorously boiling water bath for 5 min. Cool, add 10 mL of ethyl acetate, and transfer the content to a separatory funnel. With stirring, add slowly 4 mL of a 120-mg/mL solution of glacial acetic acid to the funnel. Shake the solution thoroughly and withdraw the lower layer. Transfer the upper layer to a small flask, add anhydrous sodium sulfate, and pass through a membrane disk syringe of 0.45-µm pore size. Collect the filtrate and use it as the sample.
Chromatographic system
(See Chromatography (621), Thin-Layer Chromatography.)
Mode: TLC
Plate: 10-cm x 20-cm or 20-cm x 20-cm, silica gel 60 F254
Application volume: 10 µL, as 8-mm bands. [NOTE-An automated apparatus may be used.]
Developing solvent system: Ethyl acetate, methylene chloride, methyl alcohol, and acetic acid (60:32:8:1)
Spray reagent: Prepare the anisaldehyde solution by mixing in the following order. In 0.5 mL of anisaldehyde, add 10 mL of glacial acetic acid, 85 mL of methyl alcohol, and 5 mL of sulfuric acid.
Analysis
Samples: Standard solution and Sample solution
Development: Apply the Samples in different bands to the previously marked starting point on a TLC plate, and develop the plate two times over a path of 15 cm. Dry the plate in air.
Detection: Spray with the Spray reagent. Heat the plate at 100°–105° for 10 min, and examine in daylight (or white light).
[NOTE-The principal band of aleuritic acid shows strong intensity and purple color. The retardation factor (RF) for the principal band of aleuritic acid is 0.41. A blue-gray band with medium intensity at RF 0.22 could be assigned to shellolic acid.]
Acceptance criteria: The chromatogram from the Sample solution shows several colored bands. One of the colored bands is similar in position and color to the band in the chromatogram from the Standard solution, and it is assigned to aleuritic acid. Below the aleuritic acid band, a blue-gray band is assigned to shellolic acid.
3 IMPURITIES
3.1 LIMIT OF CHLORIDE
Dilute nitric acid: Dilute 10.5 ml. of nitric acid with water to 100 mL (10%).
Silver nitrate solution: Dissolve 1.75 g of silver nitrate in water to 100 mL (0.1 mol/L).
Sample: 0.4 g
Sample solution: Shake and dissolve the Sample in 5 mL of alcohol while warming. Add 40 mL of water, and cool. Add 12 mL of Dilute nitric acid and water to make 100 mL, and filter. Perform the analysis using 50-mL of the filtrate as the Sample solution.
Control solution: 0.8 mL of 0.01 M hydrochloric acid VS, 2.5 mL of alcohol, 6 mL of Dilute nitric acid, and water to make 50 mL
Analysis: Add 1 mL of Silver nitrate solution to the Sample solution and Control solution, mix well, and protect from light for 5 min. Compare the opalescence developed in both solutions against a black background by viewing downward or transversely.
Acceptance criteria: The opalescence of the Sample solution is NMT that of the Control solution, corresponding to NMT 0.14%.
3.2 TOTAL ASH
Sample: 1 g
Analysis: Before sampling, ignite a crucible of platinum, quartz, or porcelain at 500°-550° for 1 h. Cool and weigh the crucible. Transfer the Sample to this crucible. Take off the lid or open slightly if necessary. Heat the crucible at a low temperature at first, then gradually heat to 500°-550°. Ignite to incinerate the residue for more than 4 h until no carbonized substance remains in the ash. Cool and weigh the ash. Incinerate repeatedly to constant weight, cool, weigh accurately, and determine the percentage of total ash. If a carbonized substance remains and a constant weight cannot be obtained, extract the charred mass with hot water, collect the insoluble residue on filter paper for assay, and incinerate the residue and filter paper until no carbonized substance remains in the ash. Then add the filtrate, evaporate to dryness, and incinerate. Cool, weigh accurately, and determine the percentage of the total ash. If a carbon-free ash still cannot be obtained, moisten the ash with a small amount of alcohol (ethanol). Break up the ash with a glass rod, and wash the rod with a small amount of alcohol. Evaporate carefully, and determine the mass of the total ash as described above. A desiccator (silica gel) is used for cooling.
Acceptance criteria: NMT 1.0%
3.3 ETHANOL-INSOLUBLE SUBSTANCES
Sample: 5 g
Analysis: Dissolve the Sample in 50 mL of alcohol (ethanol) in a water bath while shaking. Transfer the Ethanol solution to a tared extraction thimble that was previously dried at 105° for 2 h in a Soxhlet extractor, and extract with alcohol for 3 h. Dry the extraction thimble at 105° for 3 h. Weigh the residue in the thimble.
Acceptance criteria: The mass of the residue is NMT 2.0%.
4 SPECIFIC TESTS
4.1 LOSS ON DRYING (731)
Analysis: Dry at 41 ± 2° in a well-ventilated oven for 24 h.
Acceptance criteria: See Table 1.
4.2 ACID VALUE
Sample solution: Dissolve 2 g of finely ground Shellac in 50 mL of alcohol that has been neutralized to phenolphthalein with 0.1 N sodium hydroxide.
Analysis: Add additional phenolphthalein TS if necessary and titrate with 0.1 N sodium hydroxide VS to a pink endpoint, or determine the endpoint potentiometrically. Express the acid value in terms of the number of mg of potassium hydroxide required per g of dried Shellac, and calculate the acid value as directed in Fats and Fixed Oils (401), Acid Value.
[NOTE-For orange Shellac titrate slowly, stirring vigorously, until a glass rod dipped into the titrated solution produces a color change when touched to a drop of thymol blue TS on a spot plate.]
Acceptance criteria: See Table 1.
4.3 WAX
Sample: 10 g of finely ground Shellac
Analysis: Transfer the Sample and 2.50 g of sodium carbonate to a 200-mL, tall-form beaker. Add 150 mL of hot water, immerse the beaker in a boiling water bath, and stir until dissolved. Cover the beaker with a watch glass, and maintain the heat for more than 3 h without agitation. Remove the beaker to a cold water bath. When the wax has floated to the surface, pass the solution through medium-speed quantitative ashless filter paper, transferring the wax to the paper, and wash the filter with water. Pour 5-10 mL of alcohol onto the filter to facilitate drying. Wrap the paper loosely in a larger piece of filter paper, bind with a piece of fine wire, and dry with the aid of gentle heat. Extract with chloroform in a suitable continuous extraction apparatus for 2 h, using a weighed flask to receive the extracted wax and solvent. Evaporate the solvent, and dry the wax at 105° to constant weight.
Acceptance criteria: See Table 1.
4.4 ROSIN
Sample solution: 200 mg/mL in dehydrated alcohol
Analysis: To 10 mL of Sample solution add slowly, with shaking, 50 mL of solvent hexane, wash with two successive 50-mL portions of water, filter the washed alcohol-solvent hexane solution, and evaporate to dryness. To the residue add 2 mL of a mixture of liquefied phenol, dehydrated alcohol, and solvent hexane (1:0.5:2). Stir, and transfer a portion of the solution to the cavity of a color-reaction plate. Fill an adjacent cavity with a mixture of bromine and solvent hexane (1:4), and cover both cavities with an inverted watch glass.
Acceptance criteria: No purple or deep indigo-blue color is produced in or above the liquid containing the residue.
5 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers. Store in a dry place below 15°. Protect from light.
LABELING: Label it to indicate the type of Shellac.
USP REFERENCE STANDARDS (11)
USP Aleuritic Acid RS
USP Refined Bleached Shellac RS
USP Regular Bleached Shellac RS


