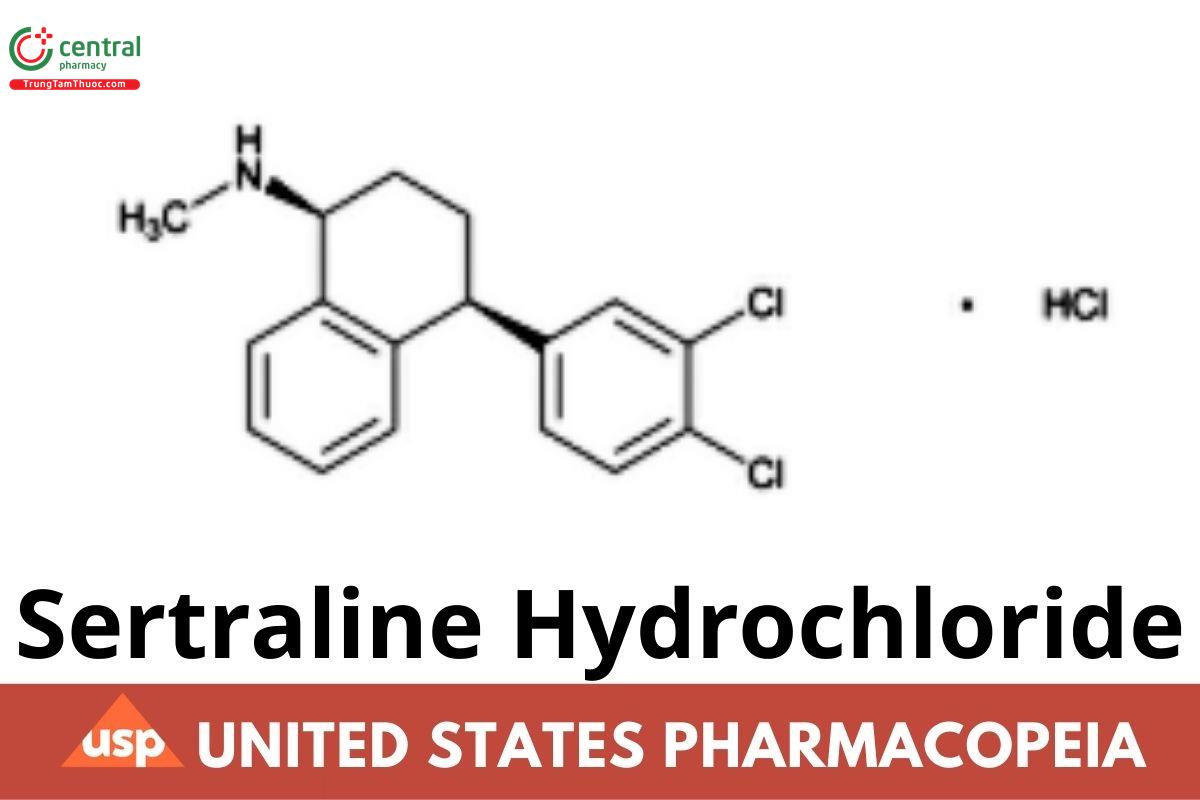
Sertraline Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
C17H17Cl2N. HCl 342.69
1-Naphthalenamine, 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-, hydrochloride, (1S-cis)-;
(1S,4S)-4-(3,4-Dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-naphthylamine hydrochloride CAS RN®: 79559-97-0; UNII: UTI8907Y6X.
1 DEFINITION
Sertraline Hydrochloride contains NLT 97.0% and NMT 102.0% of sertraline hydrochloride (C17H17Cl2N. HCl), calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197M
B. The retention time of the major peak of the Sample solution corresponds to that of sertraline hydrochloride from the System suitability solution, as obtained in the test for Limit of (R,R) Sertraline Hydrochloride.
Change to read:
C. Identification Tests—General 〈191〉, Chemical Identification Tests, Chloride
Sample solution: 1 mg/mL of Sertraline Hydrochloride in a mixture of dehydrated alcohol and water(50:50), prepared as follows. Dissolve 10 mg of Sertraline Hydrochloride in 5 mL of dehydrated alcohol. Add 5 mL of water to the solution. Acceptance criteria: Meets the requirements
3 ASSAY
Change to read:
Procedure
Buffer: To 28.6 mL of glacial acetic acid, slowly add, while stirring, 34.8 mL of triethylamine, and dilute with water to 100 mL. Dilute 10 mL of the resulting solution with water to 1 L.
Mobile phase: Acetonitrile, methanol, and Buffer (45:15:40)
Standard solution: 0.05 mg/mL of USP Sertraline Hydrochloride RS in Mobile phase
Sample solution: 0.05 mg/mL of Sertraline Hydrochloride in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 3.9-mm × 15-cm; 4-µm packing L1
Column temperature: 30°
Flow rate: 1.8 mL/min
Injection volume: 20 µL
Run time: NLT 2 times the retention time of sertraline
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 0.73%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of sertraline hydrochloride (C17H17Cl2N.HCl) in the portion of Sertraline Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Sertraline Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Sertraline Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.3%
Change to read:
Limit of (R,R) Sertraline Hydrochloride
Solution A: Transfer 10.0 mL of ammonium hydroxide to a 100-mL volumetric flask. Dilute with water to volume. Mobile phase: Chromatographic solvent hexane, 2-propanol, and diethylamine(96: 4: 0.15)
System suitability solution: Transfer 10 mg of USP Sertraline Hydrochloride Racemic Mixture RS into a 20-mL volumetric flask. Add 4 mL of Solution A and 10 mL of chromatographic solvent hexane. Shake well until the organic phase is clear. Wait for phase separation, transfer about 2.0 mL from the top layer into a 20-mL volumetric flask, and dilute with chromatographic solvent hexane to volume.
Standard solution: Transfer 10 mg of USP Sertraline Hydrochloride RS into a 20-mL volumetric flask. Add 4 mL of Solution A (USP 1-May 2021) and 10 mL of chromatographic solvent hexane. Shake well until the organic phase is clear. Wait for phase separation, transfer 1.0 mL from the top layer into a 10-mL volumetric flask, and dilute with chromatographic solvent hexane (USP 1-May 2021) to volume. Further dilute quantitatively and stepwise, if necessary, to obtain a solution having a known concentration of 0.01 mg/mL.
Sample solution: Transfer 20 mg of Sertraline Hydrochloride to a 20-mL volumetric flask. Add 4 mL of Solution A and 10 mL of chromatographic solvent hexane. Shake well until the organic phase is clear. Wait for phase separation, and use the top layer.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 235 nm
Column: 4.6-mm × 25-cm; 5-µm packing L40
Column temperature: 5°
Flow rate: 1 mL/min
Injection volume: 20 µL
Run time: NLT 4.5 times the retention time of sertraline
System suitability
Sample: System suitability solution
[Note—The relative retention times for sertraline and (R,R) sertraline are about 1.0 and 1.16, respectively.]
Suitability requirements
Resolution: NLT 2.8 between sertraline and (R,R) sertraline
Relative standard deviation: NMT 10.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of (R,R) sertraline hydrochloride in the portion of Sertraline Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of (R,R) sertraline from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Sertraline Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Sertraline Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: NMT 1.5%
Change to read:
Organic Impurities, Procedure 1
Depending on the synthetic route, perform Organic Impurities, Procedure 2 and Limit of Mandelic Acid; or perform Organic Impurities, Procedure 1. If sertralone is a known process impurity, then the tests for Organic Impurities, Procedure 2, and Limit of Mandelic Acid are recommended.
Buffer: 5.8 g/L of monobasic ammonium phosphate in water. Adjust with phosphoric acid to a pH of 4.2.
Mobile phase: Methanol and Buffer (48:52)
System suitability solution: 0.5 mg/mL each of USP Sertraline Hydrochloride RS and USP Sertraline Hydrochloride Related Compound A RS in Mobile phase
Standard solution: 0.5 µg/mL of USP Sertraline Hydrochloride RS in Mobile phase
Sample solution: 0.5 mg/mL of Sertraline Hydrochloride in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.0-mm × 25-cm; 5-µm packing L45
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: 20 µL
Run time: NLT 3 times the retention time of sertraline
System suitability
Samples: System suitability solution and Standard solution
[Note—See Table 1 for the relative retention times.]
Suitability requirements
Resolution: NLT 2.2 between sertraline hydrochloride related compound A (trans-R,S isomer) and sertraline, System suitability solution Relative standard deviation: NMT 10.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of each individual impurity in sertraline hydrochloride (C17H17Cl2N. HCl) in the portion of Sertraline Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of each individual impurity from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Sertraline Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Sertraline Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: See Table 1. The reporting threshold is 0.05%.
Table 1
Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
2,3-Isosertralinea | 0.32 | 0.15 |
4-Deschlorosertralineb | 0.42 | 0.20 |
3-Deschlorosertralinec | 0.60 | 0.20 |
Mandelic acid | 0.70 | 0.10 |
Sertraline | 1.0 | — |
Sertraline related compound A (trans-R,S isomer) | 1.19 | 0.10 |
Sertraline related compound A (trans-S,R isomer) | 1.64 | 0.10 |
Any individual unspecified impurity | — | 0.10 |
Total impurities | — | 0.5 |
a (1RS,4RS)-4-(2,3-Dichlorophenyl)-N-methyl-1,2,3,4-tetrahydronaphthalen-1-amine.
b (1RS,4RS)-4-(3-Chlorophenyl)-N-methyl-1,2,3,4-tetrahydronaphthalen-1-amine.
c (1RS,4RS)-4-(4-Chlorophenyl)-N-methyl-1,2,3,4-tetrahydronaphthalen-1-amine.
Change to read:
Organic Impurities, Procedure 2
Solution A: 250 g/L of anhydrous potassium carbonate in water
System suitability solution: Dissolve 50 mg of USP Sertraline Hydrochloride RS and 0.5 mg of USP Sertraline Hydrochloride Related Compound A RS in 2 mL of methanol in a stoppered centrifuge tube. Add 0.2 mL of Solution A, and mix in a vortex mixer for 30 s. Add 8 mL of methylene chloride. Stopper the tube, and mix in a vortex mixer for 60 s. Add 1 g of anhydrous sodium sulfate. Mix well, and centrifuge for 5 min.
Sample solution: Dissolve 250 mg of Sertraline Hydrochloride in 2 mL of methanol in a stoppered centrifuge tube. Add 0.2 mL of Solution A, and mix in a vortex mixer for 30 s. Add 8 mL of methylene chloride. Stopper the tube, and mix in a vortex mixer for 60 s. Add 1 g of anhydrous sodium sulfate. Mix well, and centrifuge for 5 min.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: GC
Detector: Flame-ionization
Column: 30-m × 0.53-mm fused silica column coated with a 1.0-µm lm of phase G3
Temperatures
Injection port: 250°
Detector: 280°
Column: See Table 2.
Table 2
Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature (°) | Hold Time at Final Temperature (min) |
200 | 0 | 200 | 1 |
200 | 2 | 260 | 8 |
Carrier gas: Helium
Flow rate: 9 mL/min
Injection volume: 1 µL
Injection type: Split; split ratio 1:10
System suitability
Sample: System suitability solution
[Note—See Table 3 for the relative retention times.]
Suitability requirements
Peak-to-valley ratio: NLT 15 between sertraline related compound A and sertraline Analysis
Sample: Sample solution
Calculate the percentage of each individual impurity in the portion of Sertraline Hydrochloride taken:
Result = (rU/rT ) × 100
rU = peak response of each individual impurity from the Sample solution
rT = sum of all the peak responses from the Sample solution
Acceptance criteria: See Table 3. The reporting threshold is 0.05%.
Table 3
Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
3,4-Deschlorosertralinea | 0.5 | 0.2 |
3-Deschlorosertralineb and 4- deschlorosertralinec | 0.7 | 0.8 |
Sertraline | 1.0 | — |
Sertraline related compound A | 1.05 | 0.2 |
Sertraloned | 1.1 | 0.2 |
Any individual unspecified impurity | — | 0.10 |
Total impuritiese | — | 1.5 |
a(1RS,4RS)-4-Phenyl-N-methyl-1,2,3,4-tetrahydronaphthalen-1-amine.
b(1RS,4RS)-4-(4-Chlorophenyl)-N-methyl-1,2,3,4-tetrahydronaphthalen-1-amine.
c(1RS,4RS)-4-(3-Chlorophenyl)-N-methyl-1,2,3,4-tetrahydronaphthalen-1-amine.
d 4-(3,4-Dichlorophenyl)-3,4-dihydronaphthalen-1(2H)-one.
e Does not include mandelic acid and 1R,4R-sertraline hydrochloride.
Change to read:
Limit of Mandelic Acid
Perform this test only if Organic Impurities, Procedure 2, is used.
Solution A: Dissolve 1 g of sodium dodecyl sulfate in 800 mL of water. Add 200 mL of acetonitrile and 1 mL of phosphoric acid.
Solution B: Dissolve 1 g of sodium dodecyl sulfate in 100 mL of water. Add 900 mL of acetonitrile and 1 mL of phosphoric acid.
Diluent: Solution A and Solution B (50:50)
Mobile phase: See Table 4.
Table 4
Time (min) | Solution A (%) | Solution B (%) |
0 | 60 | 40 |
8 | 60 | 40 |
9 | 10 | 90 |
16 | 10 | 90 |
16.1 | 60 | 40 |
20 | 60 | 40 |
System suitability solution: 0.005 mg/mL each of USP Benzoic Acid RS and USP Mandelic Acid RS in Diluent
Standard solution: 0.002 mg/mL of USP Mandelic Acid RS in Diluent
Sample solution: 1 mg/mL of Sertraline Hydrochloride in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 3-µm packing L1
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for mandelic acid, benzoic acid, and sertraline are 0.2, 0.3, and 1.0 respectively.] Suitability requirements
Resolution: NLT 5 between benzoic acid and mandelic acid, System suitability solution
Relative standard deviation: NMT 5.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of mandelic acid in the portion of Sertraline Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of mandelic acid from the Sample solution
rS = peak response of mandelic acid from the Standard solution
CS = concentration of USP Mandelic Acid RS in the Standard solution (mg/mL)
CU = concentration of Sertraline Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: NMT 0.2%
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I, Method Ia: NMT 0.50%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight, light-resistant containers at a temperature NMT 40°.
Labeling: Label to indicate the Organic Impurities procedure with which the article complies, if Organic Impurities, Procedure 1, is not used. Change to read:
USP Reference Standards 〈11〉
USP Benzoic Acid RS
USP Mandelic Acid RS
USP Sertraline Hydrochloride RS
USP Sertraline Hydrochloride Racemic Mixture RS
(1RS,4RS)-4-(3,4-Dichlorophenyl)-N-methyl-1,2,3,4-tetrahydro-1-naphthylamine hydrochloride.
C17H17Cl2N. HCl 342.69
USP Sertraline Hydrochloride Related Compound A RS
(1RS,4SR)-4-(3,4-Dichlorophenyl)-N-methyl-1,2,3,4-tetrahydro-1-naphthylamine hydrochloride.
C17H17Cl2N. HCl 342.69


