Rufinamide Tablets
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
To view the Notice from the Expert Committee that posted in conjunction with this accelerated revision, please click https://www.uspnf.com/rb-rufinamide-tabs-20220930.
1 DEFINITION
Rufinamide Tablets contain an amount of Rufinamide equivalent to NLT 95.0% and NMT 105.0% of the labeled amount of rufinamide (C10H8F2N4O).
2 IDENTIFICATION
A. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
B. The UV spectrum of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Buffer: 2.7 g/L of potassium dihydrogen phosphate in water
Diluent: Acetonitrile, methanol, and water (40:50:10)
Mobile phase: Methanol, tetrahydrofuran, and Buffer (15:5:80)
System suitability stock solution: 0.8 mg/mL of USP Rufinamide RS, and 0.02 mg/mL each of USP Rufinamide Related Compound A RS and USP Rufinamide Related Compound B RS in Diluent.
[Note—USP Rufinamide Related Compound B RS is used for identification purposes only.]
System suitability solution: 0.08 mg/mL of USP Rufinamide RS, and 2 µg/mL each of USP Rufinamide Related Compound A RS and USP Rufinamide Related Compound B RS, in Buffer from the System suitability stock solution
Standard stock solution: 0.8 mg/mL of USP Rufinamide RS in Diluent
Standard solution: 0.08 mg/mL of USP Rufinamide RS in Buffer from the Standard stock solution
Sample stock solution: Nominally 0.8 mg/mL of rufinamide in Diluent from a portion of NLT 20 finely powdered Tablets. Sonicate for 10 min, and shake for 15 min. Centrifuge a portion of the suspension.
Sample solution: Nominally 0.08 mg/mL of rufinamide in Buffer, from a portion of suspension obtained from the Sample stock solution
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 210 nm. For Identification B, use a diode array detector in the range of 190–400 nm.
Column: 4.6-mm × 12.5-cm; 5-µm packing L1
Flow rate: 1 mL/min
Injection volume: 25 µL
Run time: NLT 2.3 times the retention time of rufinamide
System suitability
Samples: System suitability solution and Standard solution
[Note—See Table 9 for relative retention times.]
Suitability requirements
Resolution: NLT 1.5 between rufinamide and rufinamide related compound A, System suitability solution
Tailing factor: NMT 1.5, Standard solution
Relative standard deviation: NMT 2.0%, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of rufinamide (C10H8F2N4O) in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Rufinamide RS in the Standard solution (mg/mL)
CU = nominal concentration of rufinamide in the Sample solution (mg/mL)
Acceptance criteria: 95.0%–105.0%
4 PERFORMANCE TESTS
Change to read:
4.1 Dissolution 〈711〉
Test 1
Medium 1: 0.1 N hydrochloric acid
Medium 2: pH 6.8 phosphate buffer
Apparatus 4: With 22.6-mm cell, glass beads in the cone, with Tablet laying on the beads. Insert 320–350 mg of glass wool in the filter insert and then a glass microfiber filter of 2.7-µm pore size and a glass microfiber filter of 0.7-µm pore size.
Times:
5 and 12 h for the 200-mg Tablets
6 and 16 h for the 400-mg Tablets
Flow rate: 16 mL/min, pulsating
Test intervals, media, and sample solutions for the 200-mg Tablets: See Table 1.
Table 1
| Samples | Interval (min) | Volume (mL) | Medium |
| 1 | 60 | 50 | 1 |
| 2 | 120 | 50 | 2 |
| 1 | 60 | 50 | 2 |
| 3 | 120 | 50 | 2 |
Test intervals (I): See Table 2.
Table 2
| Interval | Time (min) |
| I₁ | 0–60 |
| I₂ | 60–180 |
| I₃ | 180–300 |
| I₄ | 300–360 |
| I₅ | 360–480 |
| I₆ | 480–600 |
| I₇ | 600–720 |
Sample solutions (Vᵢ): See Table 3.
Table 3
| V₁ | eluate of test interval I₁; volume = 960 mL |
| V₂ to V₃ | eluate of test interval I₂ to I₃; volume = 1920 mL, each |
| V₄ | eluate of test interval I₄; volume = 960 mL |
| V₅ to V₇ | eluate of test interval I₅ to I₇; volume = 1920 mL, each |
Test intervals, media, and sample solutions for the 400-mg Tablets: See Table 4.
Table 4
| Samples | Interval (min) | Volume (mL) | Medium |
| 1 | 60 | 50 | 1 |
| 1 | 60 | 50 | 2 |
| 3 | 120 | 50 | 2 |
| 1 | 120 | 50 | 2 |
| 2 | 180 | 50 | 2 |
Test intervals (I): See Table 5.
Table 5
| Interval | Time (min) |
| I₁ | 0–60 |
| I₂ | 60–120 |
| I₃ | 120–240 |
| I₄ | 240–360 |
| I₅ | 360–480 |
| I₆ | 480–600 |
| I₇ | 600–780 |
| I₈ | 780–960 |
Sample solutions (Vᵢ): See Table 6.
Table 6
| V₁ | eluate of test interval I₁; volume = 960 mL |
| V₂ | eluate of test interval I₂; volume = 960 mL |
| V₃ to V₆ | eluate of test interval I₃ to I₆; volume = 960 mL |
| V₇ to V₈ | eluate of test interval I₇ to I₈; volume = 2880 mL, each |
Mobile phase: Water, methanol, tetrahydrofuran, and acetic acid (100:50:13:0.12), with the addition of 206 mg of sodium pentanesulfonate, monohydrate
Standard stock solution: 600 µg/mL of USP Rufinamide RS in methanol
Standard solution 1: 60 µg/mL of rufinamide in Medium 1 from the Standard stock solution
Standard solution 2: 60 µg/mL of rufinamide in Medium 2 from the Standard stock solution
Standard solution 3: 12 µg/mL of rufinamide prepared as follows. Transfer 10 mL of the Standard stock solution to a 500-mL volumetric flask, add 40 mL of methanol, and dilute with Medium 2 to volume.
Standard solution 4: 6 µg/mL of rufinamide in Medium 2 from Standard solution 3
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 25-cm; 10-µm packing L1
Flow rate: 1.2 mL/min
Injection volume: 20 µL
Run time: NLT 1.4 times the retention time of rufinamide
System suitability
Sample: Standard solution 1
Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
Analysis
Samples: Sample solutions and Standard solutions
Calculate the percentage of the labeled amount of rufinamide (C10H8F2N4O) [f(Sᵢ)] dissolved in the Sample solution (Sᵢ) by the following steps:
Calculate the regression line for the Standard solutions:
y = ax + b
y = peak area of rufinamide from the Standard solution
a = slope
x = concentration of rufinamide in the Standard solution (µg/mL)
b = y-intercept
f(Sᵢ) = [(y − b)/a] × [(Vᵢ)/(1000 × L)] × 100
y = peak area of rufinamide from the Sample solution
b = y-intercept
a = slope
Vᵢ = volume of Sample solution (mL)
L = label claim (mg/Tablet)
Cumulative percentage of the Tablet label claim dissolved:
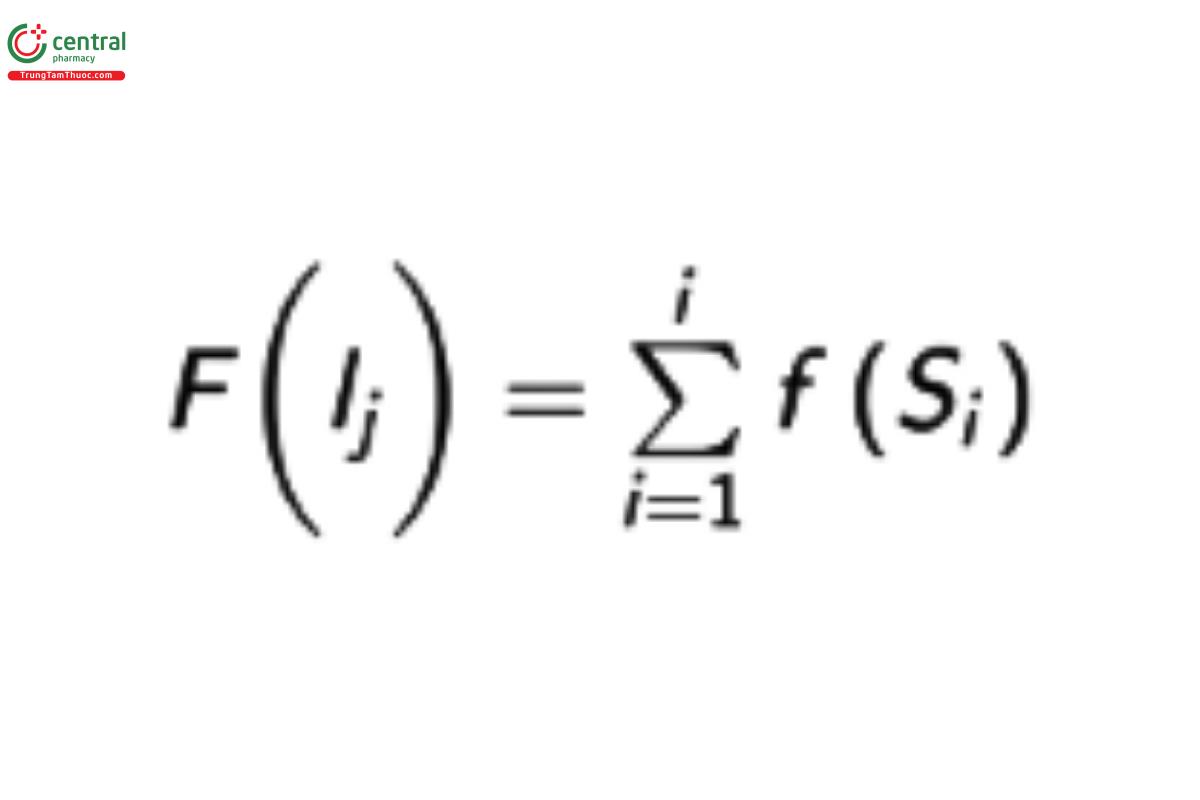
i,j = indices of test interval
Tolerances
For Tablets labeled to contain 200 mg: See Table 7.
Table 7
| Time (h) | Amount Released |
| 5 | NLT 60% |
| 12 | NLT 80% |
For Tablets labeled to contain 400 mg: See Table 8.
Table 8
| Time (h) | Amount Released |
| 6 | NLT 60% |
| 16 | NLT 80% |
The percentages of the labeled amount of rufinamide dissolved in the times specified conform to Dissolution 〈711〉, Acceptance Table 2.
Test 2: If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 2.
Medium: pH 6.8 sodium phosphate buffer containing 2% sodium dodecyl sulfate (7.8 g/L of monobasic sodium phosphate dihydrate and 0.89 g/L of sodium hydroxide in water, adjusted with phosphoric acid or 1 N sodium hydroxide VS to a pH of 6.8; to each liter of this solution add 20.0 g of sodium dodecyl sulfate and sonicate to dissolve); 2000 mL
Apparatus 2: 50 rpm
Time: 1 h for 100-mg and 200-mg Tablets; 4 h for 400-mg Tablets
Buffer: 6.8 g/L of monobasic potassium phosphate in water
Mobile phase: Acetonitrile and Buffer (30:70)
Standard stock solution: 1 mg/mL of USP Rufinamide RS in methanol
Standard solution: 0.05 mg/mL of USP Rufinamide RS from the Standard stock solution diluted with Medium
Sample solution: Pass a portion of the solution under test through a suitable filter of 0.45-µm pore size, discard the first few milliliters, and dilute with Medium if necessary.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Column temperature: 30°
Flow rate: 1.5 mL/min
Injection volume: 5 µL
Run time: NLT 2 times the retention time of the rufinamide peak
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 1.5
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of rufinamide (C10H8F2N4O) dissolved:
Result = (rU/rS) × CS × V × D × (1/L) × 100
rU = peak response of rufinamide from the Sample solution
rS = peak response of rufinamide from the Standard solution
CS = concentration of USP Rufinamide RS in the Standard solution (mg/mL)
V = volume of Medium, 2000 mL
D = dilution factor of the Standard solution
L = label claim of rufinamide (mg/Tablet)
Tolerances: NLT 80% (Q) of the labeled amount of rufinamide (C10H8F2N4O) is dissolved. (RB 16-Sep-2022)
Test 3
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 3.
Medium: pH 6.8 phosphate buffer containing 2.5% of sodium lauryl sulfate (Dissolve 6.9 g of sodium phosphate monobasic, 0.95 g of sodium hydroxide, and 25 g of sodium lauryl sulfate in 1 L of water. Adjust with phosphoric acid or 5 N sodium hydroxide to a pH of 6.8.); 2000 mL
Apparatus 2: 75 rpm
Time: 30 min
Buffer: 1.74 g/L of potassium phosphate dibasic in water. Adjust with phosphoric acid to a pH of 3.5.
Mobile phase: Acetonitrile and Buffer (30:70)
Diluent: Acetonitrile and methanol (50:50)
Standard solution: (L/2000) mg/mL of USP Rufinamide RS, where L is the label claim in mg/Tablet, prepared as follows. Transfer a suitable amount of USP Rufinamide RS to an appropriate volumetric flask. Dissolve in NMT 25% of the flask volume of Diluent using sonication. Dilute with Medium to volume.
Sample solution: Pass a portion of the solution under test through a suitable filter of 0.45-µm pore size, discarding the first 3 mL of the filtrate.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 260 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Temperature: 35°
Flow rate: 1 mL/min
Injection volume: 25 µL
Run time: NLT 2.0 times the retention time of rufinamide
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of rufinamide (C10H8F2N4O) dissolved:
Result = (rU/rS) × CS × V × (1/L) × 100
rU = peak response of rufinamide from the Sample solution
rS = peak response of rufinamide from the Standard solution
CS = concentration of USP Rufinamide RS in the Standard solution (mg/mL)
V = volume of Medium, 2000 mL
L = label claim (mg/Tablet)
Tolerances: NLT 80% (Q) of the labeled amount of rufinamide (C10H8F2N4O) is dissolved.
4.2 Uniformity of Dosage Units 〈905〉: Meet the requirements
5 IMPURITIES
5.1 Organic Impurities
Buffer, Diluent, Mobile phase, Sample stock solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
System suitability stock solution: 0.8 mg/mL of USP Rufinamide RS, and 0.02 mg/mL each of USP Rufinamide Related Compound A RS and USP Rufinamide Related Compound B RS in Diluent.
[Note—USP Rufinamide Related Compound B RS is used for identification purposes.]
System suitability solution: 0.08 mg/mL of USP Rufinamide RS, and 2 µg/mL each of USP Rufinamide Related Compound A RS and USP Rufinamide Related Compound B RS, in Buffer from the System suitability stock solution
Standard stock solution: 0.8 mg/mL of USP Rufinamide RS in Diluent
Standard solution: 0.4 µg/mL of USP Rufinamide RS from the Standard stock solution prepared as follows. Transfer a suitable volume of Standard stock solution to an appropriate volumetric flask. Add 10% of the flask volume of Diluent, and dilute with Buffer to volume.
Sensitivity solution: 0.04 µg/mL of USP Rufinamide RS from the Standard solution prepared as follows. Transfer a suitable volume of Standard solution to an appropriate volumetric flask. Add 10% of the flask volume of Diluent, and dilute with Buffer to volume.
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
[Note—See Table 9 for relative retention times.]
Suitability requirements
Resolution: NLT 1.5 between rufinamide and rufinamide related compound A, System suitability solution
Tailing factor: NMT 1.5 for rufinamide, System suitability solution
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Sample solution and Standard solution
Calculate the percentage of any individual unspecified degradation product in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of each individual unspecified degradation product from the Sample solution
rS = peak response of rufinamide from the Standard solution
CS = concentration of USP Rufinamide RS in the Standard solution (mg/mL)
CU = nominal concentration of rufinamide in the Sample solution (mg/mL)
Acceptance criteria: See Table 9. The reporting threshold is 0.05%.
Table 9
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Rufinamide | 1.0 | — |
| Rufinamide related compound A | 1.2 | — |
| Rufinamide related compound B | 1.8 | — |
| Any individual unspecified degradation product | — | 0.1 |
| Total degradation products | — | 0.5 |
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers. Store at controlled room temperature.
Labeling: When more than one Dissolution test is given, the labeling states the Dissolution test used only if Test 1 is not used.
USP Reference Standards 〈11〉
USP Rufinamide RS
USP Rufinamide Related Compound A RS
1-(2-Fluorobenzyl)-1H-1,2,3-triazole-4-carboxamide.
C10H9FN4O 220.20
USP Rufinamide Related Compound B RS
Methyl 1-(2,6-difluorobenzyl)-1H-1,2,3-triazole-4-carboxylate.
C11H9F2N3O2 253.20


