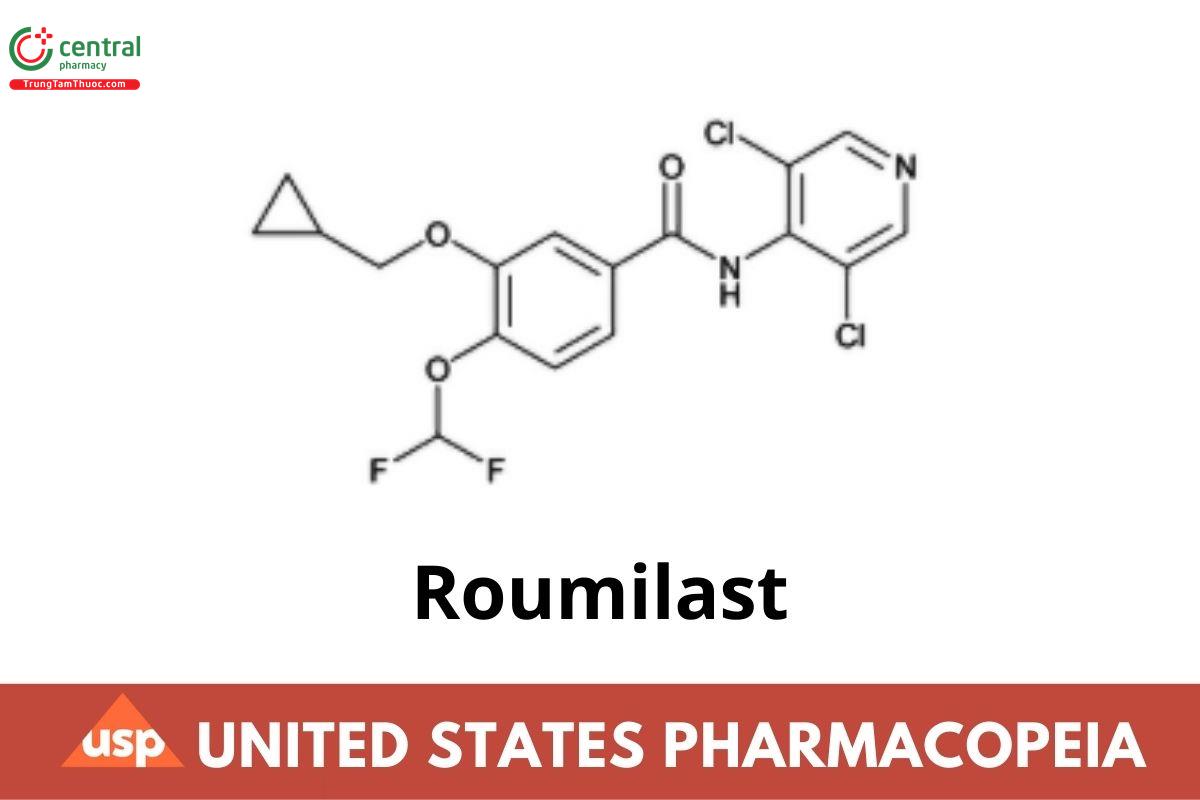
Roumilast
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Roflumilast contains NLT 98.0% and NMT 102.0% of roflumilast (C₁₇H₁₄Cl₂F₂N₂O₃), calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A or 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Protect all solutions containing roflumilast from light.
Solution A:
1.74 g/L potassium phosphate dibasic + 1.02 g/L tetrabutylammonium hydrogen sulfate in water.
Adjust to pH 5.9 with phosphoric acid.
Solution B:
1.02 g/L tetrabutylammonium hydrogen sulfate in methanol.
Mobile phase: See Table 1.
[Note—Equilibrate the LC system ≥1 h; inject solvent blank at least 3 times before analysis.]
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 75 | 25 |
| 20 | 20 | 80 |
| 26 | 20 | 80 |
| 26.1 | 75 | 25 |
| 36.1 | 75 | 25 |
System suitability solution: 0.2 mg/mL of USP Roflumilast RS and 0.001 mg/mL of USP Roflumilast Related Compound D RS in methanol
Standard solution: 0.2 mg/mL of USP Roflumilast RS in methanol
Sample solution: 0.2 mg/mL of Roflumilast in methanol
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 248 nm
Column: 2.0-mm × 12.5-cm; 5-µm packing L1.
[Note—A guard column of 2.0-mm × 1.0-cm; 5-μm packing L1 may be used.]
Column temperature: 40°
Flow rate: 0.4 mL/min
Injection volume: 10 µL
System suitability
Samples: System suitability solution and Standard solution
[Note—The relative retention times for roflumilast related compound D and roflumilast are 0.9 and 1.0, respectively.]
Suitability requirements
Resolution: NLT 2.0 between roflumilast related compound D and roflumilast, System suitability solution
Tailing factor: NMT 1.5, Standard solution
Relative standard deviation: NMT 0.85% from 6 replicate injections, Standard solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of roflumilast (C₁₇H₁₄Cl₂F₂N₂O₃) in the portion of Roflumilast taken:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × 100
Where:
rᵤ = peak response of roflumilast from the Sample solution
rₛ = peak response of roflumilast from the Standard solution
Cₛ = concentration of USP Roflumilast RS in the Standard solution (mg/mL)
Cᵤ = concentration of Roflumilast in the Sample solution (mg/mL)
Acceptance criteria: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
Residue on Ignition 〈281〉: NMT 0.2%
Organic Impurities
Protect all solutions containing roflumilast from light.
Buffer: 1.74 g/L of potassium phosphate dibasic and 1.02 g/L of tetrabutylammonium hydrogen sulfate in water. Adjust with phosphoric acid to a pH of 5.9.
Solution A: Tetrahydrofuran and Buffer (5:95)
Solution B: 1.02 g/L of tetrabutylammonium hydrogen sulfate in acetonitrile
Mobile phase: See Table 2.
[Note—A re-equilibration time of 5 min may be suitable. After each run, return to the original conditions and re-equilibrate the system.]
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 75 | 25 |
| 5 | 70 | 30 |
| 60 | 70 | 30 |
Diluent: Buffer and Solution B (50:50)
Impurity stock solution 1: 0.4 mg/mL each of USP Roflumilast Related Compound A RS, B RS, C RS, D RS, and E RS, prepared as follows. Transfer a suitable quantity of each Reference Standard to an appropriate volumetric flask and dissolve in Solution B to volume.
Impurity stock solution 2:
0.04 mg/mL each of USP Roflumilast Related Compound A RS, B RS, C RS, D RS, and E RS from Impurity stock solution 1, in Diluent
Standard stock solution:
1.0 mg/mL of USP Roflumilast RS, prepared as follows. Transfer an appropriate quantity of USP Roflumilast RS to a suitable volumetric flask, dissolve in 50% of the flask volume of Solution B, and then dilute with Buffer to volume.
Standard solution:
0.001 mg/mL of USP Roflumilast RS from the Standard stock solution, in Diluent
System suitability solution:
1.0 mg/mL of USP Roflumilast RS and 1.5 µg/mL each of USP Roflumilast Related Compounds A–E, prepared as follows. Pipet an appropriate volume of Impurity stock solution 2 into a suitable volumetric flask and dilute with Standard stock solution to volume.
Sensitivity solution:
0.3 µg/mL of USP Roflumilast RS from the Standard stock solution in Diluent
Sample solution:
1.0 mg/mL of Roflumilast prepared as follows. Transfer an appropriate quantity of Roflumilast to a suitable volumetric flask and dissolve in 50% of the flask volume of Solution B. Dilute with Buffer to volume.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 252 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L1
Column temperature: 30°
Flow rate: 1.5 mL/min
Injection volume: 30 µL
System suitability
Samples: Standard solution, System suitability solution, and Sensitivity solution
[Note—See Table 3 for the relative retention times.]
Suitability requirements:
Resolution: NLT 2.0 between roflumilast related compound B and A;
NLT 1.5 between roflumilast and compound E (System suitability solution)
Tailing factor: NMT 1.5 for roflumilast and each related compound
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of any specified and unspecified impurity:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × (1/F) × 100
Where:
rᵤ = peak response of specified or unspecified impurity from Sample solution
rₛ = peak response of roflumilast from Standard solution
Cₛ = concentration of USP Roflumilast RS in Standard solution (mg/mL)
Cᵤ = concentration of Roflumilast in Sample solution (mg/mL)
F = relative response factor (see Table 3)
Acceptance criteria: See Table 3. The reporting threshold is 0.05%.
| Name | Relative Retention Time | Relative Response Factor | Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Roflumilast related compound C | 0.06 | 1.5 | 0.15 |
| Roflumilast related compound B | 0.16 | 1.1 | 0.15 |
| Roflumilast related compound A | 0.18 | 0.87 | 0.15 |
| Roflumilast related compound D | 0.24 | 0.53 | 0.15 |
| Roflumilast | 1.00 | — | — |
| Roflumilast related compound E (a) | 1.06 | — | — |
| Total specified impurities (b) | — | — | 0.4 |
| Any individual unspecified impurity | — | — | 0.10 |
| Total unspecified impurities | — | — | 0.3 |
| Total impurities (b) | — | — | 0.60 |
(a) This impurity is listed for information only. It is controlled using the Limit of Roflumilast Related Compound E procedure and excluded from the total impurities calculation.
(b) Excluding roflumilast related compound E.
Change to read:
Limit of Roflumilast Related Compound E
[Note—Perform this test in addition to the Organic Impurities test if roflumilast related compound E is present in the drug substance due to the manufacturing process.]
Protect all solutions containing roflumilast from light.
Mobile phase: n-Hexane and dehydrated alcohol (90:10)
Impurity stock solution 1:
360 µg/mL of USP Roflumilast Related Compound E RS, prepared as follows. Transfer an appropriate quantity of USP Roflumilast Related Compound E RS to a suitable volumetric flask, dissolve in 10% of the flask volume of dehydrated alcohol, and then dilute with n-hexane to volume.
Impurity stock solution 2:
36 µg/mL of USP Roflumilast Related Compound E RS from Impurity stock solution 1 in Mobile phase
Impurity sensitivity solution:
0.3 µg/mL of USP Roflumilast Related Compound E RS from Impurity stock solution 1 in Mobile phase
Standard stock solution:
600 µg/mL of USP Roflumilast RS, prepared as follows. Transfer an appropriate quantity of USP Roflumilast RS to a suitable volumetric flask, dissolve in 10% of the flask volume of dehydrated alcohol, and then dilute with n-hexane to volume.
Standard solution:
0.6 µg/mL of USP Roflumilast RS from the Standard stock solution in Mobile phase
System suitability solution:
600 µg/mL of USP Roflumilast RS and 0.9 µg/mL of USP Roflumilast Related Compound E RS, prepared as follows. Pipet an appropriate volume of Impurity stock solution 2 to a suitable volumetric flask and dilute with Standard stock solution to volume.
Sample solution:
600 µg/mL of Roflumilast, prepared as follows. Transfer an appropriate quantity of Roflumilast to a suitable volumetric flask, dissolve in 10% of the flask volume of dehydrated alcohol, and then dilute with n-hexane to volume.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 212 nm
Column:
4.6-mm × 10-cm; 3.0-µm packing L8
Column temperature:
40°C
Flow rate:
1 mL/min
Injection volume:
8 µL
Run time:
NLT 1.8 times the retention time of roflumilast
System suitability
Samples:
Impurity sensitivity solution, Standard solution, and System suitability solution
[Note—The relative retention times for roflumilast and roflumilast related compound E are 1.0 and 1.3, respectively.]
Suitability requirements:
Resolution: NLT 2.5 between roflumilast and roflumilast related compound E, System suitability solution
Tailing factor: NMT 1.5 for roflumilast related compound E, System suitability solution
Relative standard deviation: NMT 5.0%, Standard solution
Signal-to-noise ratio: NLT 10, Impurity sensitivity solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of roflumilast related compound E in the portion of Roflumilast taken:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × (1 / F) × 100
Where:
rᵤ = peak response of roflumilast related compound E from the Sample solution
rₛ = peak response of roflumilast from the Standard solution
Cₛ = concentration of USP Roflumilast RS in the Standard solution (µg/mL)
Cᵤ = concentration of Roflumilast in the Sample solution (µg/mL)
F = relative response factor for roflumilast related compound E, 0.89
Acceptance criteria:
NMT 0.15%
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage:
Preserve in tight containers. Store at controlled room temperature. Protect from light.
USP Reference Standards 〈11〉
USP Roflumilast RS
USP Roflumilast Related Compound A RS
3-(Cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl)-4-hydroxybenzamide.
C₁₆H₁₄Cl₂N₂O₂ 353.20
USP Roflumilast Related Compound B RS
N-(3,5-Dichloropyridin-4-yl)-4-(difluoromethoxy)-3-hydroxybenzamide.
C₁₃H₈Cl₂F₂N₂O₃ 349.11
USP Roflumilast Related Compound C RS
3,5-Dichloropyridin-4-amine.
C₅H₄Cl₂N₂ 163.00
USP Roflumilast Related Compound D RS
3-(Cyclopropylmethoxy)-4-(difluoromethoxy)benzoic acid.
C₁₀H₁₀F₂O₄ 258.22
USP Roflumilast Related Compound E RS
N-(3-Bromo-5-chloropyridin-4-yl)-3-(cyclopropylmethoxy)-4-(difluoromethoxy)benzamide.
C₁₇H₁₄BrClF₂N₂O₃ 447.66


