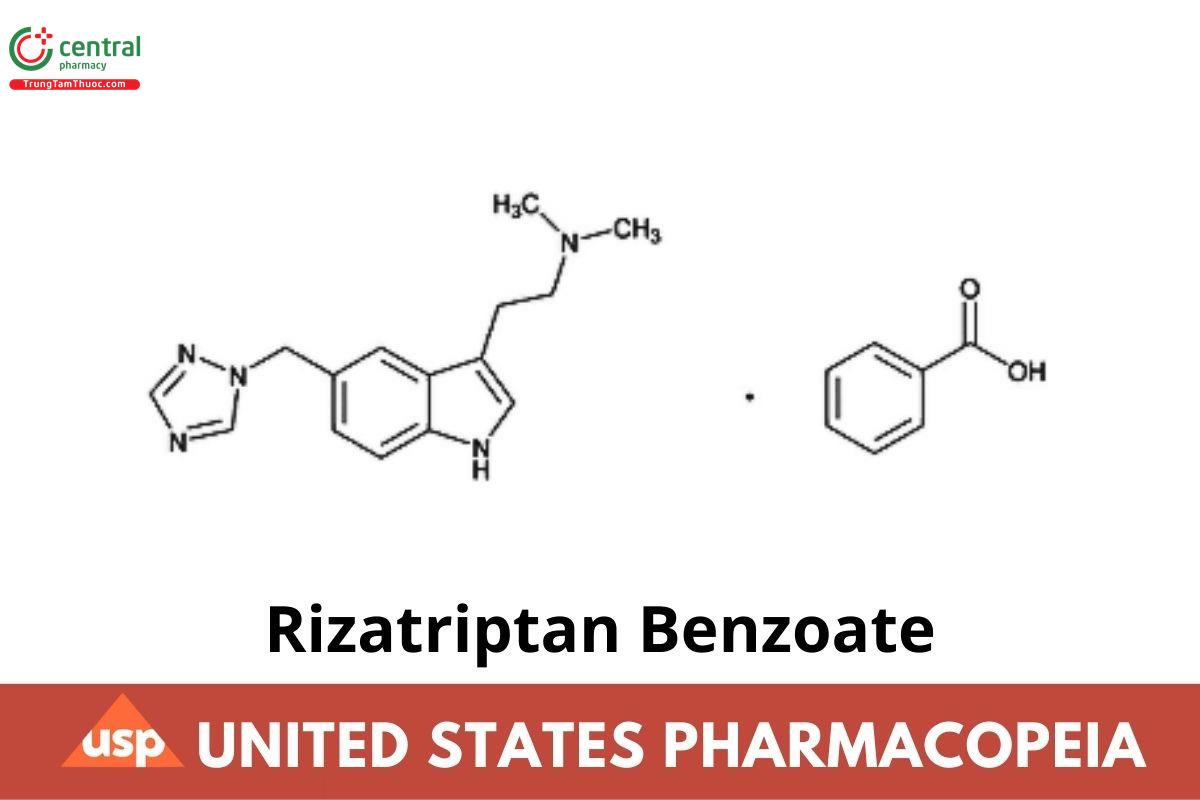
Rizatriptan Benzoate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Rizatriptan Benzoate contains NLT 98.0% and NMT 102.0% of C₂₂H₂₅N₅O₂, calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. ▲Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197A, 197K, or 197M▲ (CN 1-May-2020)
[Note—If the spectra obtained show differences, dissolve the substance to be examined and the Reference Standard separately in methanol, evaporate to dryness, and record the new spectra using the residues.]
B. The retention times of the major peaks of the Sample solution correspond to those of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
[Note—Use silanized autosampler vials and freshly prepared solutions.]
Solution A: Add 1.0 mL of trifluoroacetic acid to 1 L of a solution of acetonitrile and water (4:21), and mix.
Solution B: Acetonitrile and trifluoroacetic acid (1000:1)
Standard solution: 1 mg/mL of USP Rizatriptan Benzoate RS in Solution A
Sample solution: 1 mg/mL of Rizatriptan Benzoate in Solution A
Mobile phase: See gradient table below.
Gradient Table
| Time (min) | Solution A (%) | Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 8.0 | 100 | 0 |
| 17.0 | 70 | 30 |
| 20.0 | 70 | 30 |
| 20.1 | 100 | 0 |
| 23.0 | 100 | 0 |
Chromatographic system
Mode: LC
Detector: UV 280 nm
Column: 4.6-mm × 25-cm; 5-µm packing L11
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection size: 20 µL
[Note—The relative retention times for rizatriptan and benzoic acid are 1.0 and about 2.1.]
System suitability
Sample: Standard solution
Requirements:
Tailing factor: NMT 3.5 for rizatriptan
RSD: NMT 0.73% for the rizatriptan peak
Analysis
Samples: Standard + Sample solution
Calculate % of C₂₂H₂₅N₅O₂:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × 100
rᵤ = peak response of rizatriptan from Sample
rₛ = peak response of rizatriptan from Standard
Cₛ = concentration of USP Rizatriptan Benzoate RS (mg/mL)
Cᵤ = concentration of Rizatriptan Benzoate in Sample (mg/mL)
Acceptance criteria: 98.0%–102.0% (anhydrous)
4 IMPURITIES
Inorganic Impurities
Residue on Ignition 〈281〉: NMT 0.1%
Organic Impurities
Procedure
Solution A, Solution B, Mobile phase: As in Assay
Sample solution: 1 mg/mL Rizatriptan Benzoate in Solution A
System suitability solution: 1 mg/mL USP Rizatriptan Benzoate System Suitability Mixture RS in Solution A
Sensitivity solution: 0.5 µg/mL Rizatriptan Benzoate (by dilution of Sample with Solution A)
Chromatographic system: Same as Assay
System suitability
Samples: System suitability + Sensitivity solution
[Note—RRTs for rizatriptan, rizatriptan impurity C, benzoic acid ≈ 1.0, 1.3, 2.1]
Requirements:
Resolution: NLT 2.0 (rizatriptan vs impurity C)
S/N: NLT 10 (rizatriptan peak, Sensitivity)
Analysis
Sample: Sample solution
Result = [rᵤ / (rt − rba)] × 100
rᵤ = peak response of each impurity
rt = sum of all peaks in Sample
rba= benzoic acid peak area
Acceptance criteria:
Any individual impurity: NMT 0.10%
Total impurities: NMT 0.3%
[Disregard impurities < 0.05%.]
5 SPECIFIC TESTS
Water Determination, Method Ia 〈921〉: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage:
Store in well-closed containers at room temperature.
USP Reference Standards 〈11〉
USP Rizatriptan Benzoate RS
USP Rizatriptan Benzoate System Suitability Mixture RS
Mixture contains rizatriptan benzoate + ≥0.1% of rizatriptan impurity C.
Rizatriptan impurity C =
2-{5-[(1H-1,2,4-triazol-1-yl)methyl]-1H-indol-2-yl}-N,N-dimethylethanamine (C₁₅H₁₉N₃ 269.35)


