Rivastigmine
If you find any inaccurate information, please let us know by providing your feedback here
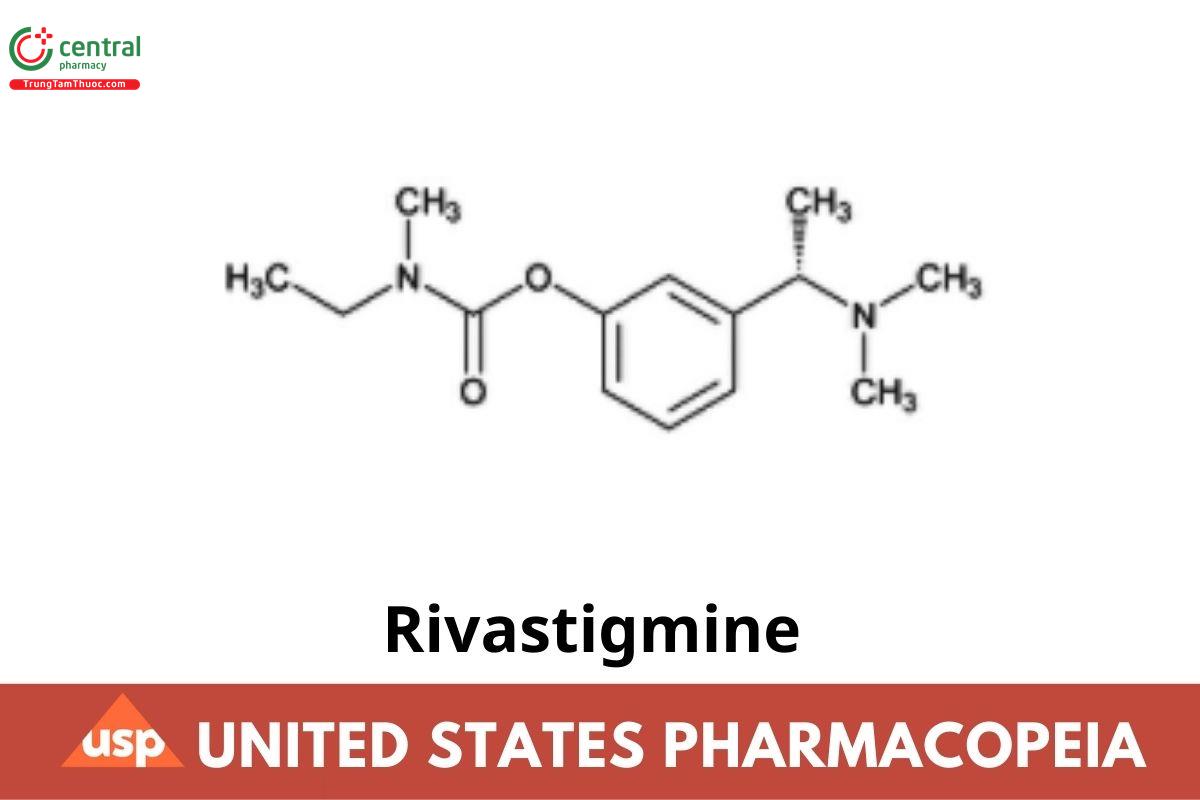
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Rivastigmine contains NLT 98.0% and NMT 102.0% of rivastigmine (C₁₄H₂₂N₂O₂), calculated on the anhydrous and solvent-free basis.
2 IDENTIFICATION
Change to read:
A. ▲Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197F▲ (CN 1-May-2020)
B. The retention time of the major peak of the Sample solution corresponds to that of the System suitability solution, as obtained in the test for Enantiomeric Purity.
3 ASSAY
Procedure
[Note—Protect solutions containing rivastigmine from light.]
Buffer: 8.9 g/L dibasic sodium phosphate dihydrate in water (0.05 M)
Mobile phase: Methanol and Buffer (58:42), adjusted to pH 8.45 with phosphoric acid
[Note—Cool to room temperature before pH adjustment.]
System suitability solution:
1 mg/mL USP Rivastigmine Tartrate RS
1 µg/mL each of:
USP Rivastigmine Related Compound B RS
USP Rivastigmine Related Compound C RS
USP Rivastigmine Related Compound D RS
in Mobile phase
Standard solution:
1.0 mg/mL USP Rivastigmine Tartrate RS in Mobile phase
Sample solution:
0.625 mg/mL Rivastigmine in Mobile phase
Chromatographic system
Mode: LC
Detector: UV 214 nm
Column: 4.0 mm × 25 cm; 5-µm L1
Column temperature: 40°
Flow rate: 1.0 mL/min
Injection volume: 20 µL
System suitability
Samples: System suitability solution and Standard solution
[Note—RRTs for related compounds C, D, B, and rivastigmine are 0.37, 0.56, 0.68, 1.0.]
Requirements:
Resolution ≥ 1.5 (compound D vs compound B)
RSD ≤ 1.0%, Standard solution
Analysis
Calculate % rivastigmine:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × (Mᵤ / Mₛ) × 100
rᵤ = peak response, Sample
rₛ = peak response, Standard
Cₛ = concentration of USP Rivastigmine Tartrate RS (mg/mL)
Cᵤ = concentration of Rivastigmine in Sample (mg/mL)
Mᵤ = molecular weight of rivastigmine = 250.34
Mₛ = molecular weight of rivastigmine tartrate = 400.42
Acceptance criteria: 98.0%–102.0% (anhydrous + solvent-free)
4 IMPURITIES
Residue on Ignition 〈281〉:
NMT 0.1%
Organic Impurities
[Note—Protect solutions from light.]
Buffer, Mobile phase, System suitability solution, Sample solution, and Chromatographic system:
As in Assay.
Standard solution:
0.0025 mg/mL USP Rivastigmine Tartrate RS
Sensitivity solution:
0.5 µg/mL Rivastigmine Tartrate RS (from Standard)
System suitability
Samples:
System suitability solution
Standard solution
Sensitivity solution
[Note—RRTs for related compounds C, D, B, rivastigmine: 0.37, 0.56, 0.68, 1.0]
Requirements:
Resolution ≥ 1.5 (compound D vs B)
S/N ≥ 10 (rivastigmine, Sensitivity solution)
RSD ≤ 10%, Standard solution
Analysis
Calculate impurity %:
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × (Mᵤ / Mₛ) × (1/F) × 100
rᵤ = impurity peak response, Sample
rₛ = rivastigmine peak response, Standard
Cₛ = concentration of Rivastigmine Tartrate RS (mg/mL)
Cᵤ = concentration of Rivastigmine in Sample
Mᵤ = 250.34
Mₛ = 400.42
F = relative response factor (Table 1)
Acceptance criteria: See Table 1
Disregard peak at RRT ~0.45.
| Name | Relative Retention Time | Relative Response Factor | NMT (%) |
|---|---|---|---|
| Phenol impurity (Compound C) | 0.37 | 1.7 | 0.3 |
| Nor impurityᵃ | 0.68 | 1.0 | 0.1 |
| Rivastigmine | 1.0 | — | — |
| Any other individual impurity | — | 1.0 | 0.10 |
| Total impurities | — | — | 0.5 |
ᵃ (S)-3-[1-(Dimethylamino)ethyl]phenyl dimethylcarbamate (racemic mixture = compound B)
Enantiomeric Purity
[Note—Protect solutions from light.]
Buffer:
1.78 g dibasic sodium phosphate dihydrate +
1.38 g monobasic sodium phosphate → dissolve → dilute to 1000 mL → pH 6.0
Mobile phase:
20.0 mL acetonitrile + 205 µL N,N-dimethyloctylamine → dilute to 1000 mL with Buffer
System suitability solution:
0.1 mg/mL Rivastigmine Tartrate RS
0.1 µg/mL R-Isomer RS
Standard solution:
0.1 µg/mL Rivastigmine Tartrate R-Isomer RS
Sample solution:
0.0625 mg/mL Rivastigmine in Mobile phase
Sensitivity solution:
0.05 µg/mL R-Isomer RS
Chromatographic system
Mode: LC
Detector: UV 200 nm
Column: 4.0 mm × 10 cm; 5-µm L41
Flow rate: 0.5 mL/min
Injection volume: 20 µL
System suitability
[Note—RRTs for R-isomer and rivastigmine: 0.85, 1.0]
Requirements:
RSD ≤ 10%, Standard solution
S/N ≥ 10, Sensitivity solution
Resolution ≥ 0.8, R-isomer vs rivastigmine
Analysis
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × (Mᵤ / Mₛ) × 100
rᵤ = peak of R-isomer, Sample
rₛ = peak of R-isomer, Standard
Cₛ = concentration in Standard (mg/mL)
Cᵤ = concentration in Sample (mg/mL)
Mᵤ = 250.34
Mₛ = 400.42
Acceptance criteria: NMT 0.3% R-enantiomer
5 SPECIFIC TESTS
Water Determination 〈921〉, Method Ia: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage:
Preserve in tight containers under inert gas, protected from light.
Store at 2°–8°.
USP Reference Standards 〈11〉
USP Rivastigmine RS
USP Rivastigmine Related Compound B RS
Nor impurity (racemic mixture)
(RS)-3-[1-(Dimethylamino)ethyl]phenyl dimethylcarbamate
C₁₃H₂₀N₂O₂ 236.31
USP Rivastigmine Related Compound C RS
Phenol impurity
(S)-3-[1-(Dimethylamino)ethyl]phenol
C₁₀H₁₅NO 165.23
USP Rivastigmine Related Compound D RS
Acetylphenol impurity
3-Acetylphenyl ethyl(methyl)carbamate
C₁₂H₁₅NO₂ 221.25
USP Rivastigmine Tartrate RS
USP Rivastigmine Tartrate R-Isomer RS
(R)-3-[1-(Dimethylamino)ethyl]phenyl ethyl(methyl)carbamate tartrate
C₁₄H₂₂N₂O₂ · C₄H₆O₆ 400.42


