Risedronate Sodium
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
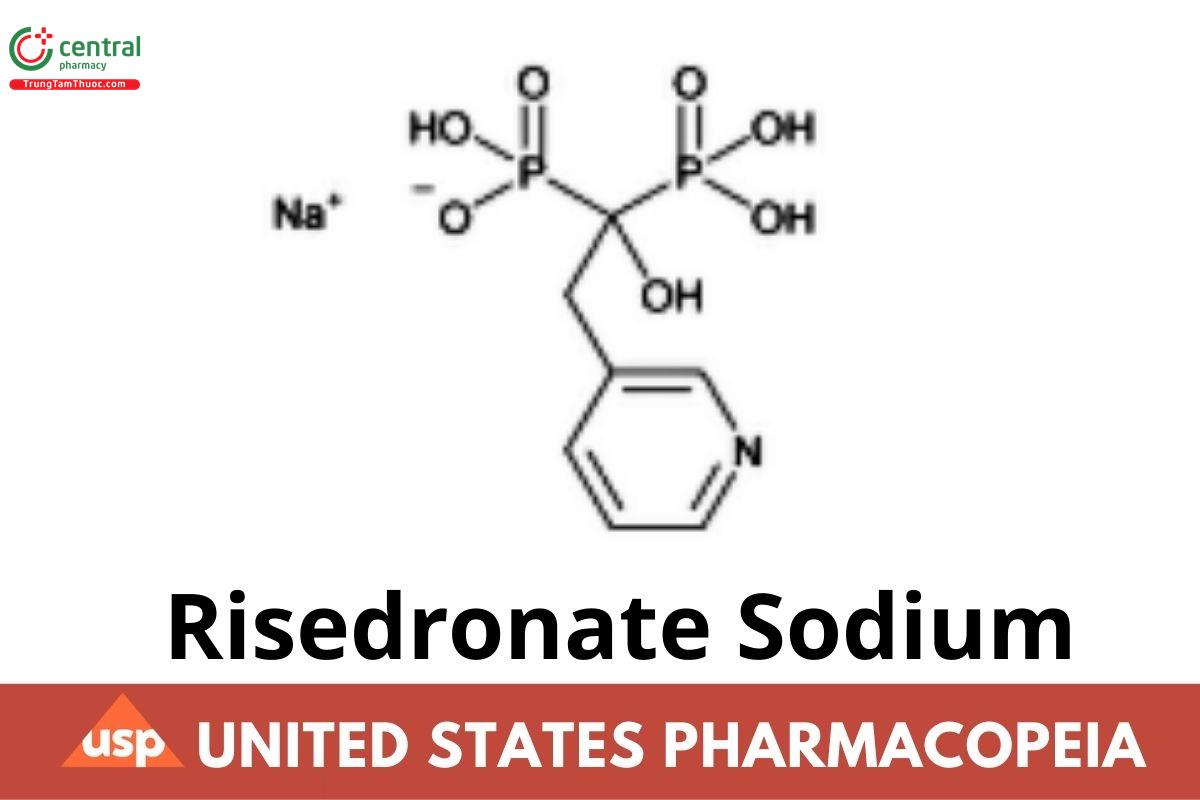
C7H10NNaO7P2 305.09
C7H10NNaO7P2 .H2O 323.12
C7H10NNaO7P2 . 2.5 H2O 350.13
Phosphonic acid, [1-hydroxy-2-(3-pyridinyl)ethylidene]bis-, monosodium salt;
Sodium trihydrogen [1-hydroxy-2-(3-pyridyl) ethylidene]diphosphonate;
Hemi-pentahydrate CAS RN®: 329003-65-8; UNII: HU2YAQ274O.
Monohydrate CAS RN®: 353228-19-0; UNII: F67L43UT5C.
1 DEFINITION
Risedronate Sodium contains one or two-and-one-half molecules of hydration. The monohydrate form contains NLT 98.0% and NMT 102.0% of risedronate sodium (C7H10NNaO7P2), calculated on the dried basis. The hemi-pentahydrate form contains NLT 98.0% and NMT 102.0% of risedronate sodium (C7H10NNaO7P2), calculated on the anhydrous basis.
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K or 197A
B. Identification Tests—General 〈191〉, Chemical Identification Tests, Sodium: Meets the requirements of test A. [Note—Complete dissolution of the sample is achieved only after the addition of the 15% potassium carbonate.]
Add the following:
C. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Change to read:
Procedure
Mobile phase: 1.8 g/L of edetate disodium in water. Adjust with 1 N sodium hydroxide to a pH of 9.5 ± 0.1.
Standard solution: 1.0 mg/mL of USP Risedronate Sodium RS and 0.1 mg/mL of USP Risedronate Related Compound A RS in Mobile phase
Sample solution: 1.1 mg/mL of Risedronate Sodium in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 263 nm
Column: 4.0-mm × 25-cm; 10-µm packing L48
Flow rate: 0.8 mL/min
Injection volume: 20 µL
System suitability
Sample: Standard solution
Suitability requirements
Resolution: NLT 2.3 between risedronate related compound A and risedronate
Tailing factor: NMT 1.6 for the risedronate peak
Relative standard deviation: NMT 1.0% for the risedronate peak
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of risedronate sodium (C7H10NNaO7P2) in the portion of Risedronate Sodium taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of risedronate from the Sample solution
rS = peak response of risedronate from the Standard solution
CS = concentration of USP Risedronate Sodium RS in the Standard solution (mg/mL)
CU = concentration of Risedronate Sodium in the Sample solution (mg/mL)
Acceptance criteria
Monohydrate: 98.0%–102.0% on the dried basis
Hemi-pentahydrate: 98.0%–102.0% on the anhydrous basis
4 IMPURITIES
Change to read:
Organic Impurities, Procedure 1
[Note—Perform both Procedure 1 and Procedure 2.]
Mobile phase, Standard solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay. Diluted standard solution: 0.005 mg/mL of USP Risedronate Sodium RS and 0.5 µg/mL of USP Risedronate Related Compound A RS in Mobile phase from the Standard solution
System suitability
Samples: Standard solution and Diluted standard solution
Suitability requirements
Resolution: NLT 2.3 between risedronate related compound A and risedronate, Standard solution
Tailing factor: NMT 1.6 for the risedronate peak, Standard solution
Relative standard deviation: NMT 1.0% for the risedronate peak, Standard solution; NMT 15% for the risedronate related compound A peak, Diluted standard solution
Analysis
Samples: Sample solution and Diluted standard solution
Calculate the percentage of each impurity in the portion of Risedronate Sodium taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of risedronate from the Diluted standard solution
CS = concentration of USP Risedronate Sodium RS in the Diluted standard solution (mg/mL)
CU = concentration of Risedronate Sodium in the Sample solution (mg/mL)
F = relative response factor (see Table 1)
Table 1
Acceptance criteria: The reporting threshold is 0.05%.
Any individual impurity: NMT 0.10%
| Name | Relative Retention Time | Relative Response Factor |
| 3-Pyridyl acetic acid | 0.22 | 1.65 |
| Risedronate related compound A (2-Pyridinyl isomer) | 0.84 | 1.0 |
| Risedronate sodium | 1.0 | — |
[Note—Disregard the peak due to the sodium ion, eluting at about 1.6 min, and any peak observed in the blank. ] Change to read:
Organic Impurities, Procedure 2
Mobile phase: Transfer 16.15 g of dibasic potassium phosphate and 0.46 g of edetate disodium to a 1-L beaker, and dissolve in about 400 mL of water. Add 1 vial of commercially available tetrabutylammonium dihydrogen phosphate buffered solution in methanol1 and 1 mL of hydrochloric acid. Adjust with 1 N sodium hydroxide or 1 N hydrochloric acid, as necessary, to a pH of 7.5 ± 0.1, and dilute with water to 480 mL. Add 20 mL of methanol, mix well, pass the solution through a nylon filter of 0.45-µm pore size, and degas.
Diluent: Transfer 0.46 g of edetate disodium to a 1-L beaker, and dissolve in 500 mL of water. Adjust with 1 N sodium hydroxide to a pH of 7.5 ± 0.1.
Standard solution: 0.005 mg/mL of USP Risedronate Related Compound B RS in Diluent
Diluted standard solution: 0.5 µg/mL of USP Risedronate Related Compound B RS in Diluent from the Standard solution Sample solution: 2 mg/mL of Risedronate Sodium in Diluent, using sonication if necessary
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 263 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Flow rate: 1.0 mL/min
Injection volume: 10 µL
System suitability
Samples: Standard solution and Diluted standard solution
Suitability requirements
Tailing factor: NMT 1.5, Standard solution
Relative standard deviation: NMT 10%, Diluted standard solution
Analysis
Samples: Standard solution and Sample solution
[Note—Disregard any peak eluting before risedronate related compound B. The risedronate peak elutes unretained at the void volume.] Calculate the percentage of each impurity in the portion of Risedronate Sodium taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
rU = peak response of each impurity from the Sample solution
rS = peak response of risedronate related compound B from the Standard solution
CS = concentration of USP Risedronate Related Compound B RS in the Standard solution (mg/mL)
CU = concentration of Risedronate Sodium in the Sample solution (mg/mL)
Mr1 = molecular weight of risedronate related compound B as a free acid, 530.20
Mr2 = molecular weight of risedronate related compound B as a tetrahydrate disodium salt, 646.22
Acceptance criteria: The reporting threshold is 0.05%.
Risedronate related compound B: NMT 0.10%
Individual impurities: NMT 0.10%
Total impurities: NMT 0.50%, Procedure 1 and Procedure 2 being combined. [Note—Disregard any peak observed in the blank. (USP 1-May 2020) ]
5 SPECIFIC TESTS
Change to read:
Water Determination 〈921〉 : Perform the test by direct introduction of solid sample into the titrator. Acceptance criteria: 11.9%–13.9%; where it is labeled as a hemi-pentahydrate
Change to read:
Loss on Drying
(See Thermal Analysis 〈891〉.)
Sample: 7–15 mg of Risedronate Sodium
Heating rate: 10°/min in a stream of nitrogen at a ow rate of about 40 mL/min
Temperature range: Ambient temperature to 250°
Acceptance criteria: 5.5%–7.5%; where it is labeled as a monohydrate
6 ADDITIONAL REQUIREMENTS
Labeling: Label to indicate whether it is the monohydrate or the hemi-pentahydrate form.
Packaging and Storage: Preserve in well-closed containers. Store at room temperature.
Change to read:
USP Reference Standards 〈11〉
USP Risedronate Sodium RS
USP Risedronate Related Compound A RS
[1-hydroxy-2-(2-pyridinyl)ethylidene]bis(phosphonic acid) monohydrate.
C7H11NO7P2 . H2O 301.13
USP Risedronate Related Compound B RS
Cyclic dimer;
Disodium tetrahydrate salt;
[3,6-Bis[(3-pyridinyl)methyl]-2,5-dihydroxy-2,5-dioxido-1,4,2,5-dioxadiphosphorinane-3,6-diyl]bis[phosphonic acid] disodium tetrahydrate salt. C14H16N2O12P4Na2 .4H2O 646.22


