Rifabutin
If you find any inaccurate information, please let us know by providing your feedback here
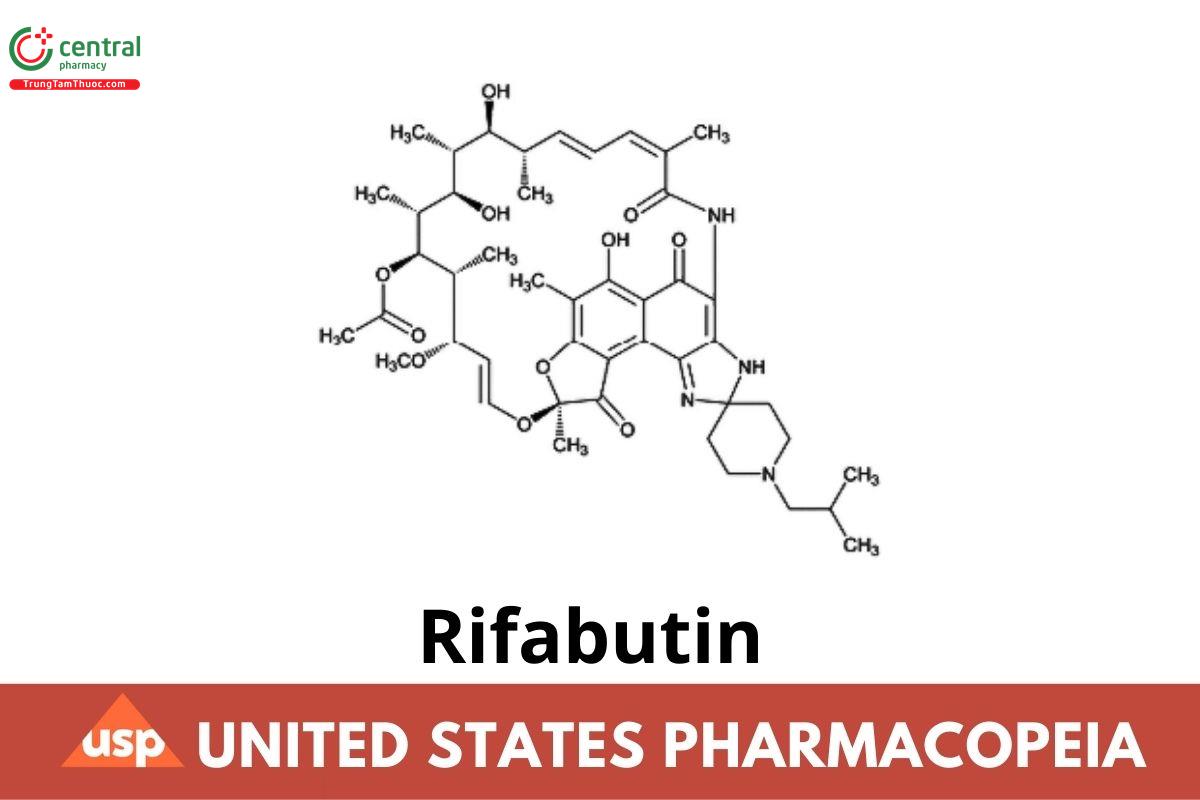
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
1 DEFINITION
Rifabutin contains NLT 950 µg/mg and NMT 1020 µg/mg of rifabutin (C₄₆H₆₂N₄O₁₁), calculated on the anhydrous basis.
2 IDENTIFICATION
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Procedure
Solution A: 13.6 g/L of monobasic potassium phosphate
Mobile phase: Acetonitrile and Solution A (50:50).
Adjust by dropwise addition of 2 N sodium hydroxide to a pH of 6.5 ± 0.1.
System suitability solution:
Dissolve about 10 mg of Rifabutin in 2 mL of methanol, add 1 mL of 2 N sodium hydroxide, stand 4 min.
Add 1 mL of 2 N hydrochloric acid and dilute with Mobile phase to 50 mL.
[Note—Portions may be stored frozen.]
Standard solution: 0.5 mg/mL USP Rifabutin RS.
Add acetonitrile to fill 10% of flask volume, dilute with Mobile phase.
Sample solution: 0.5 mg/mL Rifabutin prepared similarly.
Chromatographic system
Mode: LC
Detector: UV 254 nm
Column: 4.6 mm × 12.5 cm, 5-µm L7
Flow rate: 1 mL/min
Injection volume: 10 µL
Run time: NLT 2× rifabutin peak retention time
System suitability
System suitability solution and Standard solution.
Chromatogram of suitability solution exhibits:
major degradant peak
two minor degradant peaks
rifabutin peak
with RRT ≈ 0.5, 0.6, 0.8, 1.0.
Requirements:
Resolution ≥ 1.3 (degradant at RRT 0.8 vs rifabutin)
RSD ≤ 2.0%, Standard solution
Analysis
Calculate rifabutin (µg/mg):
Result = (rᵤ / rₛ) × (Cₛ / Cᵤ) × P
rᵤ = peak response of Sample solution
rₛ = peak response of Standard solution
Cₛ = concentration of USP Rifabutin RS (mg/mL)
Cᵤ = concentration of Rifabutin (mg/mL)
P = potency of USP Rifabutin RS (µg/mg)
Acceptance criteria: 950–1020 µg/mg (anhydrous)
4 IMPURITIES
Organic Impurities – Procedure 1 (Limit of N-Isobutylpiperidone)
Diluent: Chloroform:methanol = 1:1
Standard solution 1: 0.1 mg/mL
Standard solution 2: 0.05 mg/mL
Standard solution 3: 0.02 mg/mL
Standard solution 4: 0.01 mg/mL
Standard solution 5: 0.005 mg/mL
Sample solution: 10 mg/mL Rifabutin in Diluent
TLC system:
Adsorbent: silica gel 0.25 mm
Application: 10 µL
Solvent: hexanes:acetone (100:30)
Visualization: iodine vapor → starch TS spray.
Acceptance criteria:
NMT 0.5%.
No Sample spot at matching Rf > Standard 2.
Organic Impurities – Procedure 2
Solution A: 0.1% triethylamine in water
Solution B: ACN + A (20:80), pH 2.5 (TFA adjusted)
Solution C: ACN + A (90:10), pH 2.5
Mobile phase: Table 1.
| Time (min) | Solution B (%) | Solution C (%) |
|---|---|---|
| 0 | 75 | 25 |
| 35 | 75 | 25 |
| 45 | 45 | 55 |
| 50 | 40 | 60 |
| 60 | 30 | 70 |
| 70 | 30 | 70 |
| 71 | 75 | 25 |
Diluent: ACN:A = 20:80, pH 6.0
System suitability solution: prepared as in Assay
Standard stock solution: 1 mg/mL USP Rifabutin RS
Standard solution: 0.01 mg/mL
Sensitivity solution: 0.5 µg/mL
Sample solution: 1 mg/mL Rifabutin (prepare fresh)
Chromatographic system (Procedure 2)
Mode: LC
Detector: UV 254 nm
Column: 4.6 mm × 25 cm, 5-µm L1
Column temp: 50 °C
Flow: 1 mL/min
Injection: 20 µL
System suitability requirements:
Resolution ≥ 1.5 (rifabutin 14R vs rifabutin)
RSD ≤ 5.0%
S/N ≥ 10 (Sensitivity solution)
Analysis
% impurity = (rᵤ / rₛ) × (Cₛ / Cᵤ) × P × (F₁ / F₂) × 100
rᵤ = peak response of impurity
rₛ = peak response of Standard solution
Cₛ = concentration of USP Rifabutin RS
Cᵤ = concentration of Sample
P = potency of USP Rifabutin RS
F₁ = conversion factor (0.001 mg/µg)
F₂ = relative response factor
Reporting threshold: 0.05%
| Name | RRT | RRF | NMT (%) |
|---|---|---|---|
| 16-Desacetylrifabutinᵃ | 0.45 | 1.0 | 1.0 |
| Rifabutin 14R epimerᵇ | 0.86 | 1.0 | 0.75 |
| Rifabutin | 1.0 | 1.0 | — |
| Rifabutin N-oxideᶜ | 1.2 | 1.0 | 0.50 |
| Rifabutin 21R epimerᵈ | 1.25 | 1.0 | 0.75 |
| Didehydrorifabutinᵉ | 1.50 | 1.32 | 1.0 |
| Rifabutin N-oxide open ringᶠ | 1.60 | 1.0 | 0.25 |
| Specified unknown impurity | 1.93 | 1.0 | 0.50 |
| Any unspecified impurity | — | 1.0 | ▲0.5▲ (RB 1-Oct-2022) |
| Total impurities | — | — | 3.0 |
a (9S,12E,14S,15R,16S,17R,18R,19R,20S,21S,22E,24Z)-6,16,18,20-Tetrahydroxy-1′-isobutyl-14-methoxy-7,9,15,17,19,21,25-heptamethylspiro[9,4-(epoxypentadeca[1,11,13]trienimino)-2H-furo[2′,3′:7,8]naphtho[1,2-d]imidazole-2,4′-piperidine]-5,10,26(3H,9H)-trione.
b (9S,12E,14R,15R,16S,17R,18R,19R,20S,21S,22E,24Z)-6,18,20-Trihydroxy-1′-isobutyl-14-methoxy-7,9,15,17,19,21,25-heptamethyl-5,10,26-trioxo-3,5,9,10-tetrahydrospiro[9,4-(epoxypentadeca[1,11,13]trienimino)-2H-furo[2′,3′:7,8]naphtho[1,2-d]imidazole-2,4′-piperidine]-16-yl acetate.
c (9S,12E,14S,15R,16S,17R,18R,19R,20S,21S,22E,24Z)-16-Acetyloxy-6,18,20-trihydroxy-1′-isobutyl-14-methoxy-7,9,15,17,19,21,25-heptamethylspiro[9,4-(epoxypentadeca[1,11,13]trienimino)-2H-furo[2′,3′:7,8]naphtho[1,2-d]imidazole-2,4′-piperidine]-5,10,26(3H,9H)-trione 1′-oxide.
d (9S,12E,14S,15R,16S,17R,18R,19R,20S,21R,22E,24Z)-6,18,20-Trihydroxy-1′-isobutyl-14-methoxy-7,9,15,17,19,21,25-heptamethyl-5,10,26-trioxo-3,5,9,10-tetrahydrospiro[9,4-(epoxypentadeca[1,11,13]trienimino)-2H-furo[2′,3′:7,8]naphtho[1,2-d]imidazole-2,4′-piperidine]-16-yl acetate.
e (9S,12E,14S,15R,16S,17R,18R,19R,20S,21S,22E,24Z)-6,18,20-Trihydroxy-1′-isobutyl-14-methoxy-7,9,15,17,19,21,25-heptamethyl-21-methylene-5,10,26-trioxo-3,5,9,10-tetrahydrospiro[9,4-(epoxypentadeca[1,11,13]trienimino)-2H-furo[2′,3′:7,8]naphtho[1,2-d]imidazole-2,4′-piperidine]-16-yl acetate.
f (11R,13E,16S,17R,18S,19R,20R,21S,22S,23E,25Z)-17-Acetyloxy-6,8,11,19,21-pentahydroxy-15-methoxy-7,11,16,18,20,22,26-heptamethyl-1′-(2-methylpropyl)-5,10,27-trioxo-3,5-dihydrospiro[4,9-(epiminopentadeca[2,4,14]trienoxyethano)naphtho[1,2-d]imidazole-2,4′-piperidine] N¹′-oxide.
5 SPECIFIC TESTS
Water Determination 〈921〉, Method I: NMT 2.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage:
Preserve in well-closed containers, protected from light and excessive heat.
USP Reference Standards 〈11〉
USP Rifabutin RS


