Quinidine Gluconate
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
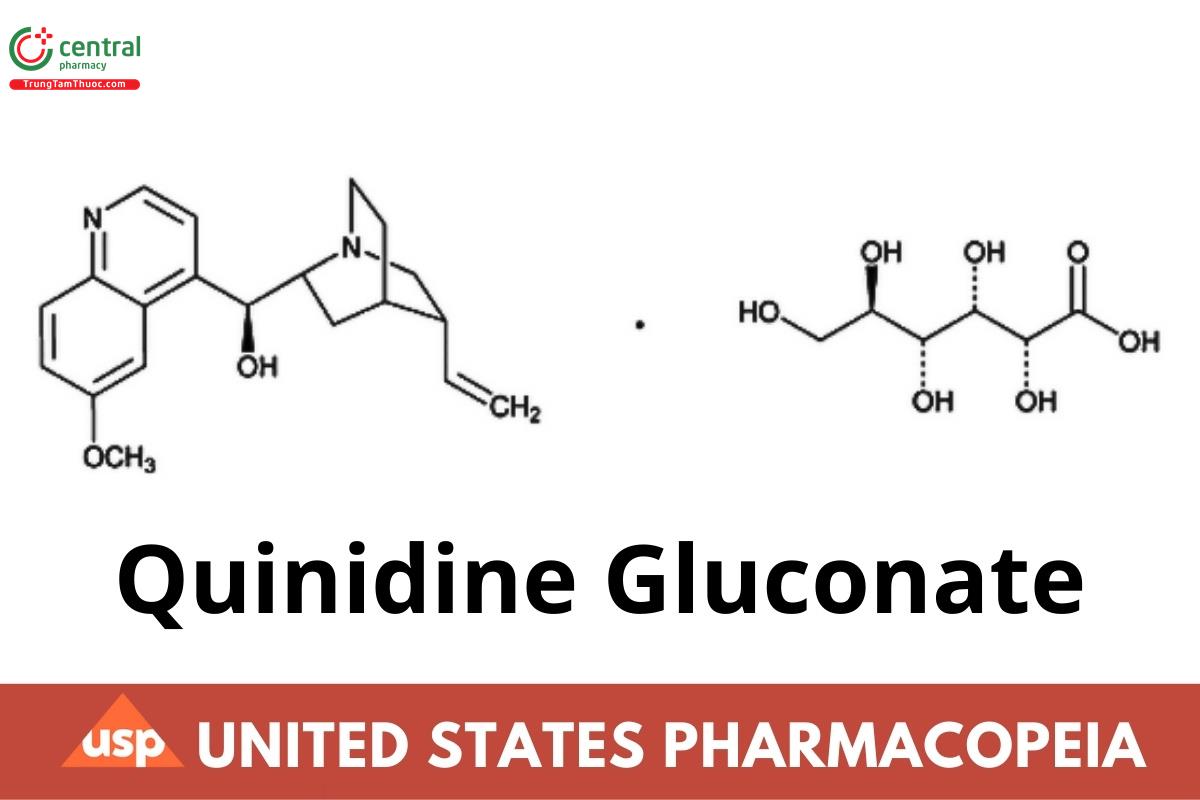
1 DEFINITION
Quinidine Gluconate is the gluconate of an alkaloid that may be obtained from various species of Cinchona and their hybrids, from Remijia pedunculata Flückiger (Fam. Rubiaceae), or prepared from Quinine. Quinidine Gluconate contains NLT 99.0% and NMT 100.5% of total alkaloid salt, calculated as quinidine gluconate (C20H24N2O2 · C6H12O7) on the dried basis.
2 IDENTIFICATION
2.1 A.
Sample solution: 1-in-2000 solution of Quinidine Gluconate in dilute sulfuric acid (1 in 350)
Acceptance criteria: The Sample solution exhibits a vivid blue fluorescence. On the addition of a few drops of hydrochloric acid, the fluorescence disappears.
2.2 B.
The R value of the principal spot of the Sample solution corresponds to that of the Standard solution, as obtained in Organic Impurities.
2.3 C.
Sample solution: 20 mg/mL of Quinidine Gluconate
Acceptance criteria: The Sample solution is dextrorotatory.
2.4 D.
Sample: Dissolve 700 mg of Quinidine Gluconate in 5 mL of water with the aid of heat. Add 1 mL of glacial acetic acid and 200 mg of phenylhydrazine hydrochloride.
Analysis: Heat in a water bath for 15 min, cool, and scratch the inner surface of the tube with a glass rod.
Acceptance criteria: Orange crystals are formed.
3 ASSAY
3.1 Procedure
Sample solution: Dissolve 150 mg of Quinidine Gluconate in 10 mL of glacial acetic acid, heating gently if necessary, and cool the solution.
Titrimetric system
Mode: Titrimetry
Titrant: 0.1 N perchloric acid VS
Endpoint detection: Visual
Analysis: To the Sample solution add 20 mL of acetic anhydride and 4 drops of p-naphtholbenzein TS, and titrate with Titrant from a 10-mL microburet to a green endpoint. Perform a blank determination, and make any necessary corrections (see Titrimetry 〈541〉). Each mL of 0.1 N perchloric acid is equivalent to 26.03 mg of total alkaloid salt, calculated as quinidine gluconate (C20H24N2O2 · C6H12O7).
Acceptance criteria: 99.0%–100.5% on the dried basis
4 IMPURITIES
4.1 Residue on Ignition 〈281〉: NMT 0.15%
4.2 Organic Impurities
Standard solution A: 6 mg/mL of USP Quinidine Gluconate RS in diluted alcohol
Standard solution B: 0.06 mg/mL of USP Quinidine Gluconate RS in diluted alcohol from Standard solution A
Standard solution C: 0.04 mg/mL of USP Quininone RS (corresponding to 0.06 mg of the gluconate) and 0.08 mg/mL of cinchonine (corresponding to 0.12 mg of the gluconate) in diluted alcohol
Sample solution: 6 mg/mL of Quinidine Gluconate in diluted alcohol
Chromatographic system
(See Chromatography 〈621〉, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 10 µL
Developing solvent system: Chloroform, acetone, and diethylamine (50:40:10)
Analysis
Samples: Standard solution A, Standard solution B, Standard solution C, and Sample solution
Proceed as directed in the chapter. The solvent chamber is used without previous equilibration. When the solvent front has moved about 15 cm, remove the plate from the chamber, mark the solvent front, and allow the solvent to evaporate. Spray with glacial acetic acid. Locate the spots on the plate by examination under long-wavelength UV light. Then spray the plate with potassium iodoplatinate TS.
Acceptance criteria: Any spot produced by the Sample solution at the R value of a spot produced by Standard solution C is not greater in size or intensity than that corresponding spot. Apart from these spots and from the spots appearing at the R value of Quinidine Gluconate and dihydroquinidine gluconate (the two spots most evident from Standard solution A), any additional fluorescent spot is not greater in size or intensity than the principal spot of Standard solution B. After treatment with potassium iodoplatinate TS, any spot produced by the Sample solution is not greater in size or intensity than a corresponding spot from Standard solution C.
4.3 Limit of Dihydroquinidine Gluconate
Solution A: Add 35.0 mL of methanesulfonic acid to 20.0 mL of glacial acetic acid, and dilute with water to 500 mL.
Solution B: Dissolve 10.0 mL of diethylamine in water to prepare a 100-mL solution.
Mobile phase: Acetonitrile, Solution A, Solution B, and water (100:20:20:860). Adjust with Solution B to a pH of 2.6.
System suitability solution: 0.2 mg/mL each of quinidine gluconate and dihydroquinidine hydrochloride prepared as follows. Dissolve a suitable quantity of each quinidine gluconate and dihydroquinidine hydrochloride in about 10% of total volume of methanol, and dilute with Mobile phase to volume.
Sample solution: 0.26 mg/mL of Quinidine Gluconate in Mobile phase
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 235 nm
Column: 3.9-mm × 30-cm; packing L1
Injection volume: 50 µL
System suitability
Sample: System suitability solution
[Note—The relative retention times for quinidine and dihydroquinidine are 1 and 1.5, respectively.]
Suitability requirements
Resolution: NLT 2.5 between quinidine and dihydroquinidine
Relative standard deviation: NMT 2.0%
Analysis
Sample: Sample solution
Acceptance criteria: 20%; the response of the dihydroquinidine peak is NMT 0.25 that of the quinidine peak.
5 SPECIFIC TESTS
Loss on Drying 〈731〉
Analysis: Dry a sample at 105° for 1 h.
Acceptance criteria: NMT 0.5%
6 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in well-closed, light-resistant containers. Store at controlled room temperature.
USP Reference Standards 〈11〉
USP Quinidine Gluconate RS
USP Quininone RS C20H22N2O2 322.40


