Quinapril Hydrochloride
If you find any inaccurate information, please let us know by providing your feedback here
Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
Change to read:
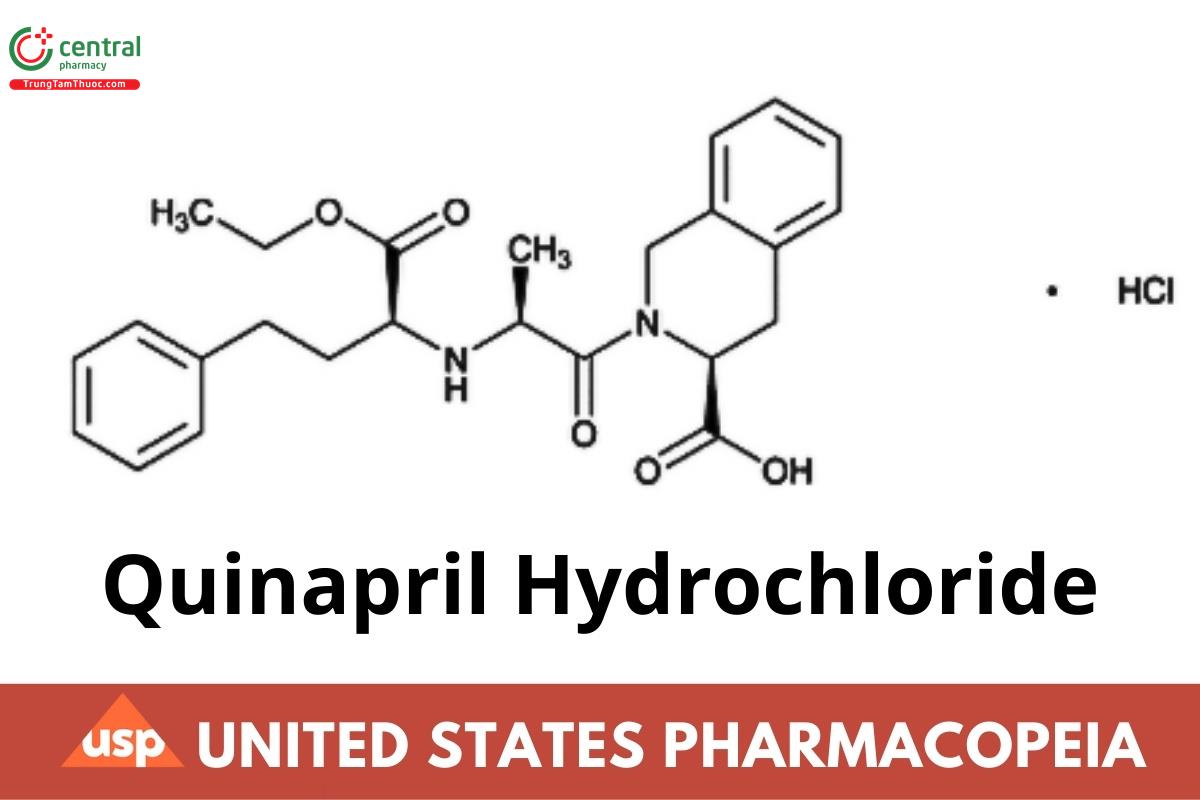
C25H30N2O5 · HCl 474.98
3-Isoquinolinecarboxylic acid, 2-[2-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-, monohydrochloride, [3S-[2[R*(R*)],3R*]];
(S)-2-[(S)-N-[(S)-1-Carboxy-3-phenylpropyl]alanyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid, 1-ethyl ester, monohydrochloride;
(S)-2-{[(S)-1-Ethoxy-1-oxo-4-phenylbutan-2-yl]-L-alanyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid hydrochloride. (USP 1-May-2021)
CAS RN®: 82586-55-8; UNII: 33067B3N2M.
Quinapril hydrochloride (acetone solvate):
C25H30N2O5 · HCl · C3H6O 533.06
CAS RN®: 757964-89-9. (USP 1-May-2021)
1 DEFINITION
Change to read:
Quinapril Hydrochloride contains NLT 98.5% and NMT 101.5% of quinapril hydrochloride (C25H30N2O5 · HCl), calculated on the anhydrous basis.
If labeled as acetone solvate, it contains NLT 98.5% and NMT 101.5% of quinapril hydrochloride (C25H30N2O5 · HCl) calculated on the anhydrous and acetone-free basis. (USP 1-May-2021)
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K or 197A (USP 1-May-2021)
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
Change to read:
3.1 Procedure
Mobile phase: Acetonitrile, methanesulfonic acid, and water (28: 0.1: 72)
Diluent: Acetonitrile and pH 6.5 0.025 M monobasic ammonium phosphate solution (40:60)
System suitability solution: 2 mg/mL of USP Quinapril Hydrochloride RS and 0.005 mg/mL each of USP Quinapril Related Compound A RS and USP Quinapril Related Compound B RS in Diluent
Standard solution: 2 mg/mL of USP Quinapril Hydrochloride RS in Diluent
Sample solution: 2 mg/mL of Quinapril Hydrochloride in Diluent
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 214 nm
Columns
Guard: 4.6-mm × 3-cm; 5-µm packing L10
Analytical: 4.6-mm × 25-cm; 5-µm packing L10
Flow rate: 1.5 mL/min
Injection volume: 10 µL
Run time: NLT 3 times retention time of quinapril (USP 1-May-2021)
System suitability
Samples: System suitability solution and Standard solution (USP 1-May-2021)
Suitability requirements
Resolution:
NLT 1.75 between quinapril and quinapril related compound A;
NLT 3.5 between quinapril and quinapril related compound B, System suitability solution
Tailing factor: NMT 2.0, Standard solution (USP 1-May-2021)
Relative standard deviation: NMT 0.55%, Standard solution (USP 1-May-2021)
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of quinapril hydrochloride (C25H30N2O5 · HCl) in the portion of Quinapril Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of quinapril from the Sample solution
rS = peak response of quinapril from the Standard solution
CS = concentration of USP Quinapril Hydrochloride RS in the Standard solution (mg/mL)
CU = concentration of Quinapril Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 98.5%–101.5% on the anhydrous basis and 98.5%–101.5% on the anhydrous and acetone-free basis if labeled as acetone solvate (USP 1-May-2021)
4 OTHER COMPONENTS
4.1 Content of Chloride
Sample: 100 mg of Quinapril Hydrochloride
Titrimetric system
(See Titrimetry 〈541〉.)
Mode: Direct titration
Titrant: 0.01 N silver nitrate VS
Analysis: Transfer the Sample to a 100-mL beaker. Dissolve in 50 mL of water and 10 mL of alcohol, and acidify with nitric acid. Titrate with Titrant using suitable electrodes. Perform a blank determination, and make any necessary corrections. Each milliliter of 0.01 N silver nitrate is equivalent to 0.3545 mg of chloride.
Acceptance criteria: 7.2%–7.6%
5 IMPURITIES
5.1 Residue on Ignition 〈281〉: NMT 0.1%
Delete the following:
5.2 Limit of Residual Solvents
Standard stock solution: Transfer 50 mL of dimethylformamide to a 200-mL volumetric flask. Add 75 mg each of acetone and acetonitrile and 30 mg each of methylene chloride and toluene, each weighed by difference. Dilute with dimethylformamide to volume.
Standard solution: 4.0 mL of the Standard stock solution, diluted with dimethylformamide to 50 mL
System suitability solution 1: Transfer 25 mL of dimethylformamide to a 50-mL volumetric flask. Add 35 µL of dehydrated alcohol and 25 µL of methylene chloride. Dilute with dimethylformamide to volume. Transfer 1.0 mL of this solution to a 50-mL volumetric flask, and dilute with dimethylformamide to volume.
System suitability solution 2: 2.0 mL of the Standard stock solution, diluted with dimethylformamide to 50 mL
Sample solution: 60 mg of Quinapril Hydrochloride to a suitable headspace vial, add 5.0 mL of dimethylformamide, seal, and shake to dissolve.
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.53 - mm * 30 - m fused silica column coated with a 1.0-µm film of phase G16 and a split injection system
Carrier gas: Helium
Flow rate: 6 mL/min
Sampler: Headspace
Vial pressure: 6.1 psi
Split flow rate: 100 mL/min (back pressure of 3.5 psi)
Injection volume: 1 mL
Temperature
Injector port: 180°
Detector: 240°
Oven temperature of the headspace sampler: 60°
Headspace loop and transfer lines: 65°
[NOTE-The vials are equilibrated for 10 min prior to injection, and injection occurs every 36 min.]
Column temperature: See the temperature table below.
| Initial Temperature (°) | Temperature Ramp (°/min) | Final Temperature(°) | Hold Time at Final Temperature (min) |
| 35 | 0 | 35 | 10 |
| 35 | 7 | 4 |
System suitability
Samples: System suitability solution 1 and System suitability solution 2
[NOTE-The relative retention times for methylene chloride and alcohol are about 0.94 and 1.0, respectively, System suitability solution 1.]
Suitability requirements
Resolution: NLT 1.2 between methylene chloride and alcohol, System suitability solution 1
Column efficiency: NLT 4900 theoretical plates, from the methylene chloride peak of System suitability solution 1
Tailing factor: NMT 1.7 for the methylene chloride peak, System suitability solution 1
Relative standard deviation: NMT 15.0%, System suitability solution 2
Analysis
Samples: Standard solution and Sample solution
Separately calculate the percentages, by weight, of acetone, acetonitrile, methylene chloride, and toluene in the portion of Quinapril Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of the relevant solvent of the Sample solution
rS = peak response of the relevant solvent of the Standard solution
CS = concentration of the relevant solvent in the Standard solution (mg/mL)
CU = nominal concentration of Quinapril Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria
Acetone: NMT 0.25%
Acetonitrile: NMT 0.25%
Methylene chloride: NMT 0.1%
Toluene: NMT 0.1% (USP 1-May-2021)
Change to read:
5.3 Organic Impurities
Mobile phase, Diluent, System suitability solution, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Sensitivity solution: 2 µg/mL of USP Quinapril Hydrochloride RS in Diluent (USP 1-May-2021)
Standard solution: 5 µg/mL each of USP Quinapril Related Compound A RS and USP Quinapril Related Compound B RS in Diluent
System suitability
Samples: System suitability solution, Sensitivity solution, and Standard solution
Suitability requirements
Resolution: NLT 1.75 between quinapril and quinapril related compound A; NLT 3.5 between quinapril and quinapril related compound B,
System suitability solution
Relative standard deviation: NMT 2.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution (USP 1-May-2021)
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of quinapril related compound A or quinapril related compound B in the portion of Quinapril Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of quinapril related compound A or quinapril related compound B from the Sample solution
rS = peak response of quinapril related compound A or quinapril related compound B from the Standard solution
CS = concentration of USP Quinapril Related Compound A RS or USP Quinapril Related Compound B RS in the Standard solution (mg/mL)
CU = concentration of Quinapril Hydrochloride in the Sample solution (mg/mL)
Calculate the percentage of any individual unspecified impurity in the portion of Quinapril Hydrochloride taken:
Result = (rU/rT) × 100
rU = peak response of any individual unspecified impurity from the Sample solution
rT = sum of the responses of all the peaks from the Sample solution
Acceptance criteria: See Table 1.
Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
| Quinapril related compound B | 0.54 | 0.5 |
| Quinapril | 1.00 | — |
| Quinapril related compound A | 1.85 | 0.5 |
| Any individual unspecified impurity | — | 0.2 |
| Total impurities | — | 2.0 |
6 SPECIFIC TESTS
Add the following:
6.1 Content of Acetone (if labeled as acetone solvate)
Solution A: Water
Solution B: Acetonitrile
Mobile phase: See Table 2.
Table 2
| Time (min) | Solution A (%) | Solution B (%) |
| 0 | 98 | 2 |
| 2.0 | 98 | 2 |
| 2.1 | 40 | 60 |
| 5.0 | 40 | 60 |
| 5.1 | 98 | 2 |
| 7.0 | 98 | 2 |
Standard solution: 1.0 mg/mL of USP Acetone RS in water prepared as follows. Weigh and transfer an appropriate amount of USP Acetone RS to a suitable volumetric flask containing 50% of the final volume of water. Dilute with water to volume.
Sample solution: 10 mg/mL of Quinapril Hydrochloride in water. Sonicate if necessary.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 264 nm
Column: 4.6-mm × 10-cm; 2.6-µm packing L1
Temperatures
Autosampler: 20°
Column: 35°
Flow rate: 2.0 mL/min
Injection volume: 50 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 1.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of acetone in the portion of Quinapril Hydrochloride as acetone solvate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of acetone from the Sample solution
rS = peak response of acetone from the Standard solution
CS = concentration of USP Acetone RS in the Standard solution (mg/mL)
CU = concentration of Quinapril Hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 7.9%–13.9% (USP 1-May-2021)
6.2 Water Determination 〈921〉, Method I: NMT 1.0%
6.3 Optical Rotation 〈781S〉, Procedures, Specific Rotation
Sample solution: 20 mg/mL in methanol
Acceptance criteria: +14.4° to +15.4°
7 ADDITIONAL REQUIREMENTS
Change to read:
Packaging and Storage: Preserve in well-closed containers, and store at controlled room temperature. If labeled as an acetone solvate, store in a tight container filled with nitrogen at controlled room temperature. (USP 1-May-2021)
Add the following:
Labeling: Where it is an acetone solvate form, the label so indicates. (USP 1-May-2021)
Change to read:
USP Reference Standards 〈11〉
USP Acetone RS (USP 1-May-2021)
USP Quinapril Hydrochloride RS
USP Quinapril Related Compound A RS
Ethyl [3S-[(2R*),3a,11ab]]-1,3,4,6,11,11a-hexahydro-3-methyl-1,4-dioxo-α-(2-phenylethyl)-2H-pyrazino[1,2-b]isoquinoline-2-acetate;
Also known as Ethyl (S)-2-{(3S,11aS)-3-methyl-1,4-dioxo-1,3,4,6,11,11a-hexahydro-2H-pyrazino[1,2-b]isoquinolin-2-yl}-4-phenylbutanoate. (USP 1-May-2021)
C25H28N2O4 420.51
USP Quinapril Related Compound B RS
3-Isoquinolinecarboxylic acid, 2-[2-[(1-carboxy-3-phenylpropyl)amino]-1-oxopropyl]-1,2,3,4-tetrahydro-, [3S-[2[R*(R*)],3R*]];
Also known as (S)-2-{[(S)-1-Carboxy-3-phenylpropyl]-L-alanyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid. (USP 1-May-2021)
C23H26N2O5 410.47


