Pseudoephedrine Hydrochloride Extended-Release Tablets
If you find any inaccurate information, please let us know by providing your feedback here

Tóm tắt nội dung
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
To view the Notice from the Expert Committee that posted in conjunction with this accelerated revision, please click https://www.uspnf.com/rb-pseudoephedrine-hcl-ert-20190222
1 DEFINITION
Pseudoephedrine Hydrochloride Extended-Release Tablets contain NLT 90.0% and NMT 110.0% of the labeled amount of pseudoephedrine hydrochloride (C10H15NO . HCl).
2 IDENTIFICATION
Change to read:
A. Spectroscopic Identification Tests 〈197〉, Infrared Spectroscopy: 197K
Sample: Triturate a number of Tablets, nominally equivalent to 180 mg of pseudoephedrine hydrochloride. Filter with about 10 mL of chloroform collected using vacuum filtration. Maintain the vacuum until no further filtrate can be collected, and evaporate the chloroform on a steam bath, taking care to avoid overheating. Recrystallize the residue from a small amount of dehydrated alcohol.
Acceptance criteria: Meet the requirements
B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
3 ASSAY
3.1 Procedure
Mobile phase: Alcohol and ammonium acetate solution (1 in 250) (850:150). Filter, and degas
Standard solution: 1.2 mg/mL of USP Pseudoephedrine Hydrochloride RS in alcohol
Sample stock solution: Transfer NLT 20 Tablets to a suitable container, add 500 mL of alcohol, and homogenize until the Tablets are dispersed. Quantitatively transfer the contents of the container to a 1000-mL volumetric flask, dilute with alcohol to volume, mix, and allow to stand for the solids to settle.
Sample solution: Transfer 25.0 mL of the supernatant from the Sample stock solution into a 50-mL volumetric flask, dilute with alcohol to volume, and mix. Pass a portion of this solution through a filter of 0.45-µm pore size.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; packing L3
Flow rate: 0.7 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.5
Relative standard deviation: NMT 2.0%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of pseudoephedrine hydrochloride (C10H15NO . HCl) in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response from the Sample solution
rS = peak response from the Standard solution
CS = concentration of USP Pseudoephedrine Hydrochloride RS in the Standard solution (mg/mL)
CU = nominal concentration of pseudoephedrine hydrochloride in the Sample solution (mg/mL)
Acceptance criteria: 90.0%–110.0%
4 PERFORMANCE TESTS
Change to read:
4.1 Dissolution 〈711〉
For Tablets labeled for dosing every 12 h
Test 1
Medium: Water; 900 mL
Apparatus 2: 50 rpm
Times: 1, 3, and 6 h
Mobile phase and System suitability: Proceed as directed in the Assay.
Standard solution: 0.13 mg/mL of USP Pseudoephedrine Hydrochloride RS in water
Sample solution: Filter a portion of the solution under test.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 15-cm; packing L3
Flow rate: 0.7 mL/min
Injection volume: 50 µL
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of the labeled amount of pseudoephedrine hydrochloride (C10H15NO . HCl) dissolved.
Tolerances: See Table 1.
Table 1
| Time (h) | Amount Dissolved (%) |
| 1 | 25–45 |
| 3 | 50–75 |
| 6 | NLT 75 |
The percentages of the labeled amount of pseudoephedrine hydrochloride (C10H15NO . HCl) dissolved at the times specified conform to Dissolution 〈711〉, Acceptance Table 2.
Test 3
Medium: Water; 900 mL
Apparatus 2: 50 rpm
Times: 1, 3, and 6 h
Standard solution: A known concentration of USP Pseudoephedrine Hydrochloride RS in Medium
Sample solution: Pass portions of the solution under test through a filter of 0.45-µm pore size, and suitably dilute with Medium.
Analysis: Calculate the percentage of the labeled amount of pseudoephedrine hydrochloride (C10H15NO . HCl) dissolved by comparing the maximum absorbance of Sample solution with that of Standard solution at about 214 nm.
Tolerances: See Table 2.
Table 2
| Time (h) | Amount Dissolved (%) |
| 1 | 25–45 |
| 3 | 60–80 |
| 6 | NLT 80 |
Test 4
Medium, Apparatus, and Times: Proceed as directed in Test 1.
Solution A: Acetonitrile and water (45:55)
Mobile phase: 2.5 g/L of Docusate sodium in Solution A. Add 1.0 mL of phosphoric acid. Adjust with ammonia water, 25% to a pH of 3.2.
Standard solution: (L/900) mg/mL of USP Pseudoephedrine Hydrochloride RS in Medium, where L is the label claim in mg/Tablet.
Sample solution: Withdraw a portion of the solution under test from each vessel at the specified time point and pass through a suitable filter of 0.45-µm pore size. Replace the portion of solution withdrawn with an equal volume of Medium previously equilibrated to 37.0° ± 0.5°.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 215 nm
Column: 4.6-mm × 15-cm; 5-µm packing L1
Column temperature: 40°
Flow rate: 1.5 mL/min
Injection volume: 10 µL
Run time: NLT 1.5 times the retention time of pseudoephedrine
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 2.0%
Analysis
Calculate the concentration (Cᵢ) of pseudoephedrine hydrochloride (C10H15NO . HCl) in the sample withdrawn at each time point i:
Result = (rU/rS) × CS
rU = peak response of pseudoephedrine from the Sample solution
rS = peak response of pseudoephedrine from the Standard solution
CS = concentration of USP Pseudoephedrine Hydrochloride RS in the Standard solution
Calculate the percentages of the labeled amount dissolved at each time point i:
Result1 = C1 × V × (1/L) × 100
Result2 = [(C2 × V) + (C1 × VS)] × (1/L) × 100
Result3 = {(C3 × V) + [(C2 + C1) × VS]} × (1/L) × 100
Ci = concentration of pseudoephedrine hydrochloride in the portion of sample withdrawn at time point i (mg/mL)
V = volume of the Medium, 900 mL
L = label claim (mg/Tablet)
VS = volume of the Sample solution withdrawn at each time point (mL)
Tolerances: See Table 3.
Table 3
| Time Point (i) | Time (h) | Amount Dissolved (%) |
| 1 | 1 | 27–47 |
| 2 | 3 | 53–73 |
| 3 | 6 | NLT 80 |
The percentages of the labeled amount of pseudoephedrine hydrochloride (C₁₀H₁₅NO·HCl) dissolved at the times specified conform to Dissolution 〈711〉, Acceptance Table 2. (RB 1-Mar-2019)
For Tablets labeled for dosing every 24 h
Test 2
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 2.
Medium: 0.9% sodium chloride in water; 50 mL
Apparatus 7 (see Drug Release 〈724〉): 30 cycles/min; 2–3 cm amplitude. To prepare the sample, see Figure 1.
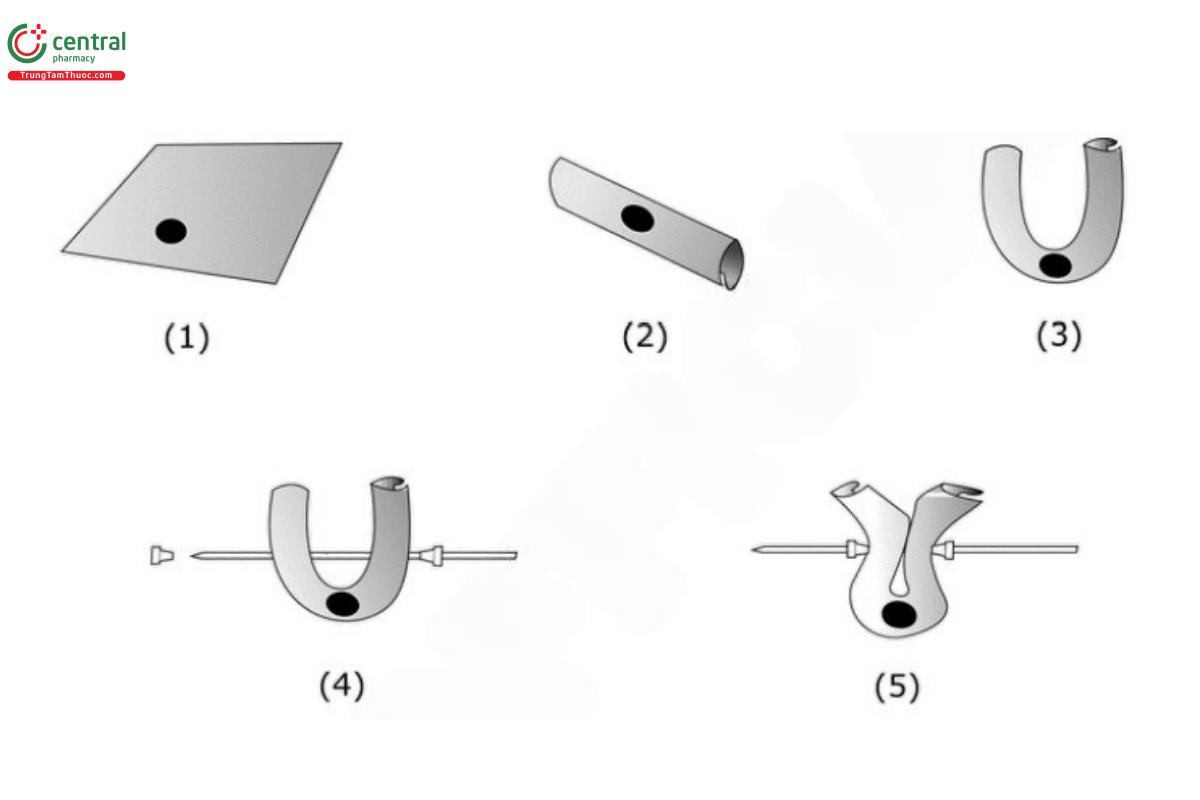
Figure 1. Steps to prepare the sample.
(1) Place 1 Tablet on a 5-cm × 5-cm nylon netting.
(2) Fold the netting over the Tablet. Continue folding until the Tablet is enclosed in the netting.
(3) Fold the netting so that the two open ends meet. The Tablet should be enveloped in the center of the netting.
(4) Insert a rod (see Drug Release 〈724〉, ▲Figure 5c▲) through the netting to secure the Tablet.
(5) Secure the netting with HPLC plastic ferrules or other appropriate device. Trim the excess netting. Attach each sample holder to the vertically reciprocating sample holder.
Times: 2, 8, 14, and 24 h
Solution A: Transfer 200 mL of water to a 1000-mL volumetric flask. Add 3.4 mL of phosphoric acid and 5 mL of triethylamine. Add water to almost 900 mL. Adjust with 1 N sodium hydroxide to a pH of about 6.8, dilute with water to volume, and mix.
Mobile phase: Methanol and Solution A (100:900)
System suitability solution: 0.4 mg/mL of USP Pseudoephedrine Hydrochloride RS in water
Sample solution: Solution under test
Standard solution: Known concentrations of USP Pseudoephedrine Hydrochloride RS in water, in a range around the expected concentration of the Sample solution at each time interval.
Chromatographic system
(See Chromatography 〈621〉, System Suitability.)
Mode: LC
Detector: UV 254 nm
Column: 4.6-mm × 5-cm; packing L1
Flow rate: 1.5 mL/min
Injection volume: 10 µL
System suitability
Sample: System suitability solution
Suitability requirements
Tailing factor: NMT 2
Relative standard deviation: NMT 2.0%
Analysis
Samples: Sample solution and Standard solution
Measure the major peak responses of the Standard solution and Sample solution. Construct a calibration curve by plotting the peak response versus concentrations of the Standard solution. Determine the amount of pseudoephedrine hydrochloride (C10H15NO . HCl) dissolved at each time interval from a linear regression analysis of the calibration curve.
Tolerances: See Table 4 (RB 1-Mar-2019).
Table 4 (RB 1-Mar-2019)
| Time (h) | Amount Dissolved (%) |
| 2 | 20–35 |
| 8 | 40–65 |
| 14 | 60–90 |
| 24 | NLT 85 |
The percentages of the labeled amount of pseudoephedrine hydrochloride (C10H15NO . HCl) dissolved at the times specified conform to Dissolution 〈711〉, Acceptance Table 2.
Uniformity of Dosage Units 〈905〉: Meet the requirements
5 ADDITIONAL REQUIREMENTS
Packaging and Storage: Preserve in tight containers.
Labeling: When more than one Dissolution test is given, the labeling states the Dissolution test used only if Test 1 is not used.
USP Reference Standards 〈11〉
USP Pseudoephedrine Hydrochloride RS


