Propylene Glycol Monocaprylate
If you find any inaccurate information, please let us know by providing your feedback here
This article is compiled based on the United States Pharmacopeia (USP) – 2025 Edition
Issued and maintained by the United States Pharmacopeial Convention (USP)
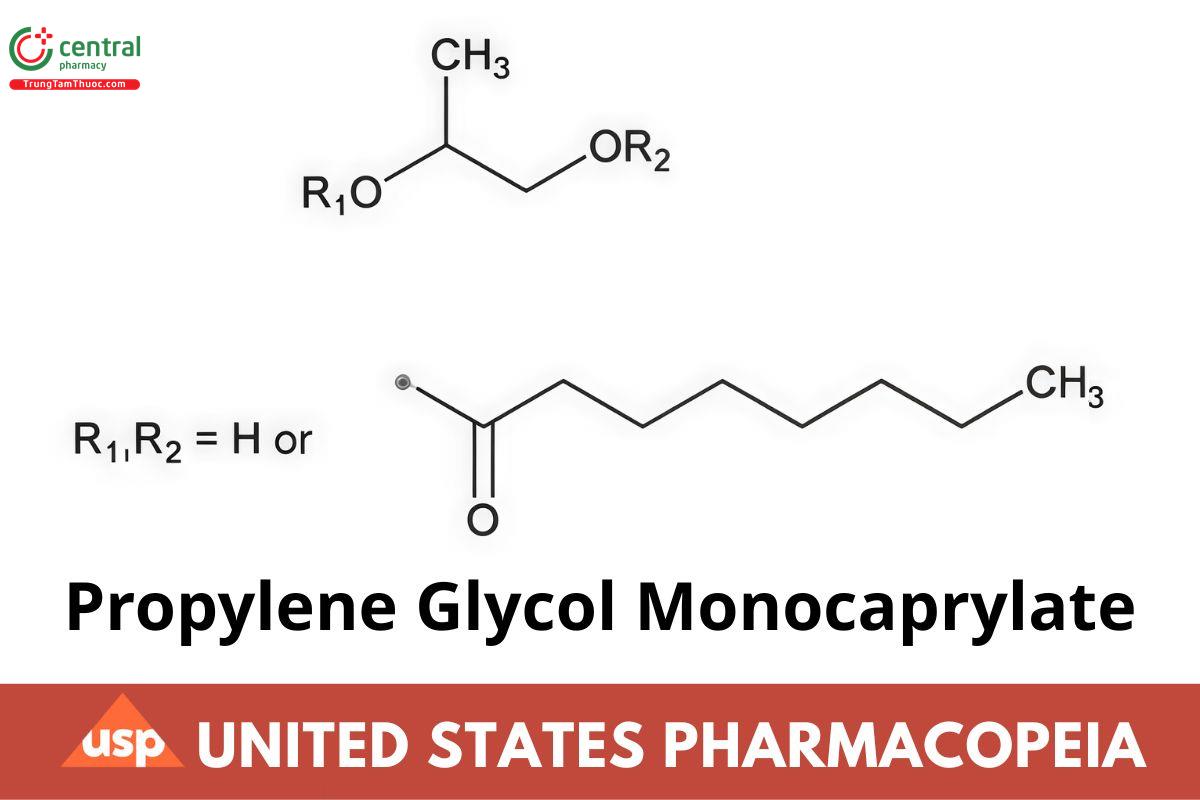
C11H22O3 202.29
(propylene glycol monocaprylate)
C19H3604 328.49
(propylene glycol dicaprylate)
Octanoic acid, ester with 1,2-propanediol;
Hydroxypropyl octanoate CAS RN®: 31565-12-5.
1 DEFINITION
Propylene Glycol Monocaprylate is a mixture of the propylene glycol monoesters and diesters of fatty acids composed predominantly of caprylic acid. Propylene Glycol Monocaprylate contains NLT 90.0% monoesters and NMT 10.0% diesters.
2 IDENTIFICATION
A. SPECTROSCOPIC IDENTIFICATION TESTS (197), Infrared Spectroscopy: 197A
B. THIN-LAYER CHROMATOGRAPHIC IDENTIFICATION TEST (201)
Standard solution: 50 mg/mL of USP Propylene Glycol Monocaprylate RS in methylene chloride
Sample solution: 50 mg/mL of Propylene Glycol Monocaprylate in methylene chloride
Chromatographic system
Application volume: 10 µL
Developing solvent system: Ether and hexane (70:30)
Spray reagent: 0.1 mg/mL of rhodamine 6G in alcohol
Analysis: Develop the chromatogram over a path of 15 cm, and dry the plate in a current of air. Spray the plate with Spray reagent, and locate the spots on the plate by examination under UV light at a wavelength of 365 nm.
Acceptance criteria: The R values of the principal spots from the Sample solution correspond to those from the Standard solution.
C. It meets the requirements in Specific Tests for Fats and Fixed Oils (401), Procedures, Fatty Acid Composition.
3 ASSAY
PROCEDURE
Mobile phase: Tetrahydrofuran
Sample solution: 40 mg/mL of Propylene Glycol Monocaprylate in Mobile phase
Chromatographic system
(See Chromatography (621), System Suitability.)
Mode: LC
Detector: Refractive index
Column: 7-mm x 60-cm; 5-µm packing L21 (100 A). [NOTE-TWO 7-mm x 30-cm; 5-µm packing L21 columns may be used in place of one 60-cm column, provided System suitability requirements are met.]
Temperatures
Column: 40°
Detector: 40°
Flow rate: 1 mL/min
Injection volume: 40 µL
System suitability
Sample: Sample solution
[NOTE-The relative retention times with reference to propylene glycol for diesters and monoesters are about 0.85 and 0.90, respectively.]
Suitability requirements
Relative standard deviation: NMT 2.0% for the monoester peak
Analysis
Sample: Sample solution
Calculate the percentage of monoesters or diesters in the portion of Propylene Glycol Monocaprylate taken:
Result = (rU/rT) x (100 - D)
rU = peak response for monoesters or diesters
rT = sum of the peak responses of the monoesters and diesters
D = sum of the percentage content of propylene glycol and the percentage content of free fatty acids
Calculate the percentage content of free fatty acids:
Result = (A/561.1) x 144
A = acid value
Acceptance criteria
Monoesters: NLT 90.0%
Diesters: NMT 10.0%
4 IMPURITIES
LIMIT OF PROPYLENE GLYCOL
Mobile phase, Sample solution, and Chromatographic system: Proceed as directed in the Assay.
Standard stock solution: 4 mg/mL of USP Propylene Glycol RS in Mobile phase
Standard solutions: Into four 5-mL volumetric flasks, introduce 0.25, 0.5, 1.0, and 2.5 mL of Standard stock solution, respectively, and dilute with Mobile phase to volume. In a fifth 5-mL volumetric flask, introduce 5.0 mL of Standard stock solution.
Analysis
Samples: Standard solutions and Sample solution
Prepare a standard curve of peak area versus concentration, in mg/mL, of propylene glycol in the Standard solutions. Obtain the concentration of propylene glycol in the Sample solution from the standard curve.
Calculate the percentage of free propylene glycol in the portion of Propylene Glycol Monocaprylate taken:
Result = (C/CU) x 100
C = concentration of propylene glycol in the Sample solution from the standard curve
CU = concentration of the Sample solution (mg/mL)
Acceptance criteria: NMT 1.5%
5 SPECIFIC TESTS
FATS AND FIXED OILS (401), Procedures, Acid Value: NMT 1.5
FATS AND FIXED OILS (401), Procedures, Fatty Acid Composition: Propylene Glycol Monocaprylate exhibits the composition profile of fatty acids shown in Table 1.
Table 1
| Carbon-Chain | Length Number of Double Bonds | Percentage (%) |
| 8 | 0 | ≥90.0 |
| 10 | 0 | ≤3.0 |
| 12 | 0 | ≤3.0 |
| 14 | 0 | ≤3.0 |
| 16 | 0 | ≤1.0 |
FATS AND FIXED OILS (401), Procedures, Jodine Value: NMT 1.0
FATS AND FIXED OILS (401), Procedures. Saponification Value: 270-295
FATS AND FIXED OILS (401), Procedures, Peroxide Value: NMT 6.0.
WATER DETERMINATION (921), Method I, Method la
Analysis: Use a mixture of methanol and methylene chloride (1:1) in place of methanol in the titration vessel.
Acceptance criteria: NMT 1.0%
ARTICLES OF BOTANICAL ORIGIN (561), Methods of Analysis, Total Ash: NMT 0.1%
6 ADDITIONAL REQUIREMENTS
PACKAGING AND STORAGE: Preserve in well-closed containers and protect from moisture. No storage requirements are specified.
USP REFERENCE STANDARDS (11)
USP Propylene Glycol RS
USP Propylene Glycol Monocaprylate RS
Also known as USP Propylene Glycol Monocaprylate Type II RS


